2984
HR-MAS 1H-NMR investigations of ovine Achilles tendon and rat rotator cuff tendon1Department of Radiology and Imaging Sciences, Emory University, Atlanta, GA, United States, 2Department of Chemistry, Georgia Institute of Technology, Atlanta, GA, United States, 3Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, GA, United States
Synopsis
Recent work quantifying tendon chemical shift information has reported resonances consistent with proteoglycan and lipid chemical groups. Currently, there is a lack of prior NMR studies of tendon available to interpret these signals and make assignments to matrix constituents. HR-MAS 1H-NMR is an ideal tool for resolving chemical composition and structure in semisolid tissues like tendon. This study reports 1H-NMR spectral assignments using 1D and 2D HR-MAS experiments in ex vivo tendon samples. Preliminary results show peaks attributed to residues comprising collagen fibrils, proteoglycans, lipids, and lactate. Ongoing work seeks to characterize 1H NMR signatures of healthy and injured tendon.
Introduction
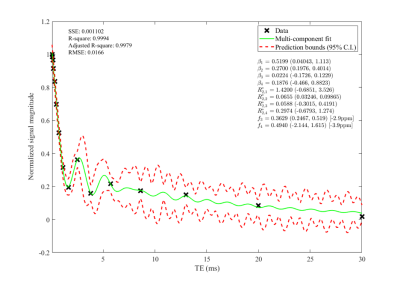
Tendons are an important load-bearing tissue in the musculoskeletal system. whose matrix composition is closely associated with its functional status. Tendons consist primarily of collagen fibrils surrounded by non-collagenous extracellular proteins and lipids. These proteins can be grouped into proteoglycans, glycoproteins, and glycoconjugates1,2,3. The location, nature, and function of these non-collagenous matrix components have been less well characterized, and ongoing work examines how these matrix components influence tendon functional properties. Tendon injury can also result in lipid infiltration within the extracellular matrix2,3. Furthermore, in vivo quantitative UTE relaxation measurements in tendon demonstrate chemical shift signals consistent with PG and lipid resonances4 (Figure 1). In this study, high-resolution magic angle spinning (HR-MAS) NMR spectroscopy is used to characterize the chemical composition of ex vivo tendon samples. HR-MAS reduces line broadening in semi-solid tissue, like tendon, by suppressing dipolar couplings, chemical anisotropy, and magnetic susceptibility at the solid-liquid interface, allowing for a better resolved spectrum. The purpose of this study is to use 1D and 2D HR-MAS 1H-NMR spectroscopy to better characterize tendon matrix components, allowing for a clearer interpretation of in vivo UTE chemical shift signals and their interpretation as it relates to tendon matrix integrity.Methods
Five ovine Achilles tendon samples and four rat rotator cuff tendon samples obtained from a subscapular transection injury model were excised and placed into HR-MAS rotor inserts with D2O for a field-lock frequency reference. 1H NMR experiments were recorded on a Bruker spectrometer operating at 400.13 MHz, with a MAS frequency of 5000 Hz. The spectra were recorded at a temperature of 298K and chemical shifts were recorded with respect to D2O. One-dimensional 1H spectral width was 20 ppm acquired with a solvent saturation. For two-dimensional spectra, COSY parameters were: spectral width (f2) = 13.0 ppm, 2048 complex points; spectral width (f1) = 13.0 ppm, 128 t1 increments with 64 scans per t1 value; repetition delay = 2.0 s, and HSQC parameters were: spectral width (f2) = 13.0 ppm, 2048 complex points; spectral width (f1) = 220 ppm, 256 t1 increments with 64 scans per t1 value; repetition and evolution delay = 1.5 s and 0.2 ms, respectively. MNOVA software (Mestrelab, Spain) was used to process the data and peaks were auto-selected. Peak assignments were made by comparing chemical shifts to those found in literature and on the Biological Magnetic Resonance Bank (BMRB).Results
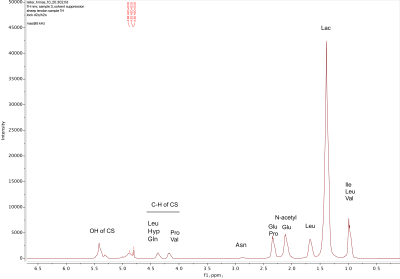
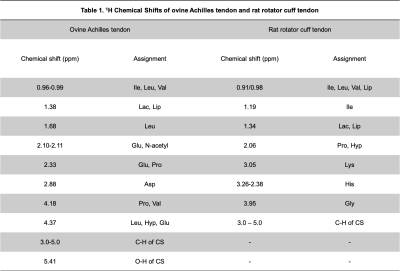
In the 1D 1H spectra (Figure 2), the largest peak amplitude in both ovine and rat tendon is observed at ~1.3 ppm and is assigned to lactate/lipid (presence of doublet is not discernable at this scale)5-10. Multiple peaks ranging between 0.96 and 0.99 ppm are attributed to isoleucine and leucine and also overlap with terminal –CH3 from fatty acids5-10. 2D HSQC spectra that provide 13C chemical shift couplings allow for discrimination between lipid and amino acid resonances, showing, for the ovine Achilles tendon, that these peaks are attributed to PG residues. In contrast, in injured rat tendon samples, HSQC spectra indicate these resonances to be attributed to lipids. Peaks ranging between 3.0-5.0 ppm are attributed to C-H resonances of chondroitin sulfate (or dermatan sulfate), which comprise the glycosaminoglycan (GAG) chains of PG8. A characteristic peak attributed to the N-acetyl group of chondroitin sulfate is observed at ~2.0 ppm8, which is seen predominantly in ovine Achilles tendon but is found at a lower intensity in rat rotator cuff tendon.Discussion
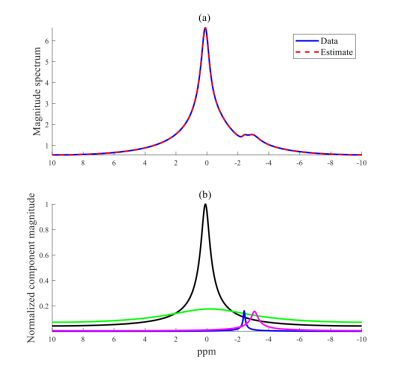
The substantial peak amplitude of lactate/lipid is of interest since quantitation of lactate in tendon isn’t well-studied. Lactate, a product of anaerobic glycolysis, has been shown to stimulate collagen production in tenocytes in a hypoxic environment following injury, and lactate concentrations have been observed to remain elevated as damaged tissue returns to its normoxic state due to the conversion of TCA intermediates into lactate11,12. Thus, the presence of lactate may indicate prior tissue degradation or repair. Lipid infiltration and proteoglycan breakdown have also been attributed to tendon pathologies, and quantification of each of these, as done by HR-MAS, will allow for better characterization of tendon status. The presence of lipids in ovine and rat tendon is likely a consequence of imposed injury. Comparing the chemical shifts of the 1H MAS spectra with those of non-MAS 1H spectra of ovine Achilles tendon (Figure 3) shows comparable resonances at ~2.2 ppm and ~1.3 ppm. These resonances are similar to those observed in a multi-component analysis of UTE data from human Achilles tendon (Figure 1), with resonances observed at -2.9 ppm and -3.9 ppm relative to water at 0 ppm; given the resonance of water as ~4.8 ppm, these chemical shifts are determined to be ~1.9 ppm and ~0.9 ppm.Conclusion
HR-MAS allows for detailed investigation into the origins of off-resonance MRI signals, providing an effective tool for interpreting resonances in UTE imaging as it relates to changes in tendon composition. Ongoing work is focused on determining the extent to which these MRI-visible resonances, observed in UTE images of tendon, can be used as quantitative biomarkers for assessing tendon structural integrity and metabolism during injury.Acknowledgements
No acknowledgement found.References
1. Fullerton, G. D., & Rahal, A. (2007). Collagen structure: The molecular source of the tendon magic angle effect. Journal of Magnetic Resonance Imaging, 25(2), 345–361.
2. Screen, H. R. C., Berk, D. E., Kadler, K. E., Ramirez, F., & Young, M. F. (2015). Tendon Functional Extracellular Matrix. Journal of Orthopaedic Research, 33(6), 793–799.
3. Thorpe, C. T., Birch, H. L., Clegg, P. D., & Screen, H. R. C. (2013). The role of the non-collagenous matrix in tendon function. International Journal of Experimental Pathology, 94(4), 248–259.
4. Anjum, M. A. R., Gonazalez, F. M, Swain, A., Leisen, J., Hosseini, Z., Singer, A., Umpierrez, M., Reiter, D.A. (2020) Multi-component T2* relaxation modelling in human Achilles tendon: Quantifying chemical shift information in ultra-short echo time imaging. Magnetic Resonance in Medicine. (Manuscript accepted on December 15, 2020)
5. Borel, M., Pastoureau, P., Papon, J., Madelmont, J. C., Moins, N., Maublant, J., & Miot-Noirault, E. (2009). Longitudinal profiling of articular cartilage degradation in osteoarthritis by high-resolution magic angle spinning 1H NMR spectroscopy: Experimental study in the meniscectomized guinea pig model. Journal of Proteome Research, 8(5), 2594–2600.
6. Li, J., Vosegaard, T., & Guo, Z. (2017). Applications of nuclear magnetic resonance in lipid analyses: An emerging powerful tool for lipidomics studies. Progress in Lipid Research, 68(September), 37–56.
7. Ling, W., Regatte, R. R., Schweitzer, M. E., & Jerschow, A. (2008). Characterization of bovine patellar cartilage by NMR. NMR in Biomedicine, 21(3), 289–295.
8. Mucci, A., Schenetti, L., & Volpi, N. (2000). 1H and 13C nuclear magnetic resonance identification and characterization of components of chondroitin sulfates of various origin. Carbohydrate Polymers, 41(1), 37–45.
9. Schiller, J., Naji, L., Huster, D., Kaufmann, J., & Arnold, K. (2001).1H and 13C HR-MAS NMR investigations on native and enzymatically digested bovine nasal cartilage. Magma (New York, N.Y.), 13(1), 19–27.
10. Schiller, Jürgen, Huster, D., Fuchs, B., Naji, L., Kaufmann, J., & Arnold, K. (2004). Evaluation of cartilage composition and degradation by high-resolution magic-angle spinning nuclear magnetic resonance. Methods in Molecular Medicine, 101(1), 267–285.
11. Comstock, J. P., & Udenfriend, S. (1970). Effect of lactate on collagen proline hydroxylase activity in cultured L-929 fibroblasts. Proceedings of the National Academy of Sciences of the United States of America, 66(2), 552–557. https://doi.org/10.1073/pnas.66.2.552
12. Hunt, T. K., Conolly, W. B., Aronson, S. B., & Goldstein, P. (1978). Anaerobic metabolism and wound healing: An hypothesis for the initiation and cessation of collagen synthesis in wounds. The American Journal of Surgery, 135(3), 328–332. https://doi.org/10.1016/0002-9610(78)90061-2
13. Anjum, M. A. R., Pawel A. Dmochowski, and Paul D. Teal. "A subband Steiglitz‐McBride algorithm for automatic analysis of FID data." Magnetic Resonance in Chemistry 56.8 (2018): 740-747.
Figures