2977
3D sub-millimeter isotropic knee cartilage T1ρ mapping using multi-interleaved fluid-attenuated TSE acquisition (MIXTURE)1Department of Radiology, Saitama Medical University, Saitama, Japan, 2Philips Japan, Tokyo, Japan
Synopsis
For T1ρ quantification, a three-dimensional (3D) acquisition is desired to obtain high-resolution images. In this study, we introduced a new sequence, termed MIXTURE (Multi-Interleaved X-prepared TSE with inTUitive RElaxometry), based on 3D magnetization-prepared multi-interleaved TSE, to achieve rapid and robust acquisition of 3D T1ρ mapping data. MIXTURE might be useful for improving the quality and efficiency of 3D T1ρ-mapping.
Introduction
Spin-lattice relaxation in the rotating frame (T1ρ) has been shown to be sensitive to biochemical changes such as proteoglycan content of articular cartilage1. Conventional T1ρ-weighted imaging protocols often consist of two-dimensional (2D) anisotropic resolution sequences, which can be time consuming, and have relatively thick sections and gaps between sections that can lead to partial-volume artifacts. Three-dimensional (3D) isotropic resolution sequences can reduce partial-volume artifacts through the acquisition of thin continuous sections through joints2. Furthermore, the isotropic source data can be used to create multiplanar reformations (MPRs), thereby eliminating the need to repeat sequences in multiple planes. The use of 3D isotropic resolution sequences in clinical practice could markedly decrease examination times, which would improve patient comfort, reduce motion artifacts, and increase the clinical efficiency of the MR imaging unit.Recently, a unique interleaved spin-lock (SL) prepared rapid gradient echo sequence has been proposed3,4 to achieve 3D sub-millimeter (0.8mm3) isotropic T1ρ-mapping of the whole knee-joint in 10 minutes using, which employs different SL pulses alternately before each gradient-echo train for improving motion-robustness. Although this approach generates high quality 3D T1ρ maps and comparable T1ρ values with literature3,4, there is still a problem that the relative signal intensity at different SL preparation times depends not only on T1ρ exponential decay due to gradient echo readout, hence phase cycling approach is recommended1. However, the phase cycling doubled total acquisition time, it is not feasible for clinical examination. On the other hand, turbo spin-echo (TSE)-based T1rho is also proffered as an alternative of phase cycling GRE approach1. By using TSE readout, the relative image intensity at different SL preparation times depends only on T1ρ exponential decay during T1rho prep, and no phase cycling is needed, it makes TSE based T1ρ imaging highly SNR efficient. Furthermore, TSE is basically less sensitive to magnetic susceptibility effects, hence TSE might adapt for any patient conditions, such as metal implants.
In this study, we introduced a new sequence, termed MIXTURE (Multi-Interleaved X-prepared TSE with inTUitive RElaxometry), based on 3D magnetization-prepared multi-interleaved TSE, to achieve rapid and robust acquisition of 3D T1ρ mapping data. The purpose of this study was to demonstrate the feasibility of accelerated 3D sub-millimeter isotropic T1ρ-mapping of the knee-joint using MIXTURE.
Methods
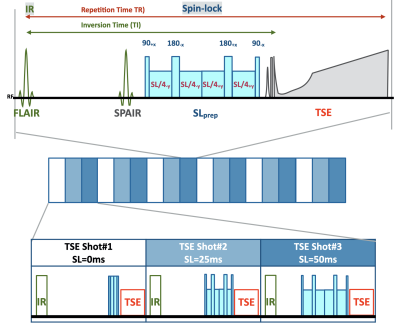
A total of 6 patients were examined on a 3.0T system (Ingenia Elition, Philips Healthcare). The study was approved by the local IRB, and written informed consent was obtained from all subjects.Scheme of the MIXTURE sequence for 3D fluid-attenuated T1ρ-mapping is shown in Figure 1. A segmented 3D TSE with variable refocusing flip angles was employed similar to a conventional MSK 3D TSE scan. T1ρ mapping was performed using an inversion-recovery and SL-prepared segmented TSE sequence with spectral fat suppression. Inversion-recovery was used for suppression of synovial fluid. T1 weighted fluid-attenuated TSE (T1FLAIR) T1rho approach is used in several neuro applications5,6, we followed this method for knee T1rho mapping. The sequence has 3 interleaved TSE shot-blocks to acquire 3 images with different SL preparation times (SL = 0, 25, and 50ms) [Fig.2]. Amplitude of the SL pulse was set to 500 Hz. Double-refocusing spin-lock pulses7 were applied for improving the robustness of SL pulses across the entire imaging volume.
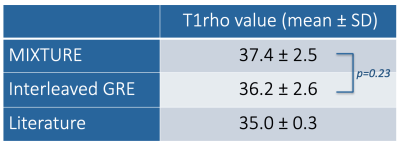
To validate the obtained T1ρ values, sagittal T1ρ maps were quantitatively compared. The average T1ρ values and their standard deviations (SDs) in the region-of-interests (ROIs) of the knee cartilage were used for comparison with previous GRE-based 3D sequence3,4. We also compared with the T1ρ values reported in literature8.
Imaging parameters for MIXTURE T1p mapping were: sagittal, TR=8.5ms, TE=3.5ms, TFE factor=60, voxel size=0.8mm3, inversion delay=860ms, SPAIR, C-SENSE reduction factor=5, and total acquisition time=8:42, and for GRE-based sequence were: sagittal, TR=2000ms, TE=26ms, TSE factor=128, voxel size=0.8mm3 TFE shot interval=5000ms, inversion delay=1760ms, ProSet1331, C-SENSE reduction factor=6, and total acquisition time=10:57.
Results and Discussion
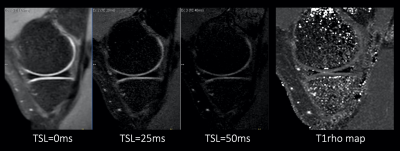
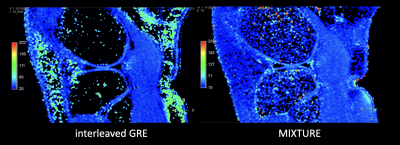
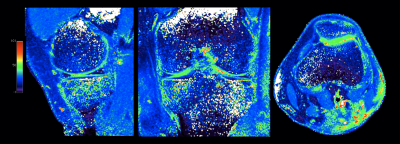
Comparison of T1ρ values with SDs between previous interleaved GRE sequence3,4 and MIXTURE measured at the central lateral portion of the femur was shown in Table 1. T1ρ values of both MIXTURE and interleaved GRE sequence were close to the values reported in literature8. There was no significant difference between MIXTURE and interleaved GRE sequence. Figure 3 shows the comparison images of T1ρ-mapping with interleaved GRE sequence and MIXTURE. Representative MPR images of MIXTURE T1ρ-mapping with 0.8mm3 acquisition were shown in Figure 4. The optimized sequence provided motion-insensitive high-quality isotropic images less than 9 minutes.Conclusion
The study demonstrated the feasibility of rapid 3D sub-millimeter (0.8mm3) isotropic T1ρ-mapping of the whole knee-joint using 3D TSE-based approach. This technique might be useful for improving the quality and efficiency of 3D T1ρ-mapping.Acknowledgements
No acknowledgement found.References
1. Wang YXJ, et al. T1ρ magnetic resonance: basic physics principles and applications in knee and intervertebral disc imaging. Quant Imaging Med Surg. 2015 Dec;5(6):858-85.
2. Pakin SK, et al. 3D-T1ρ quantitation of patellar cartilage at 3.0T. J Magn Reson Imaging. 2006 Dec; 24(6): 1357-63.
3. Nagawa K, et al. Rapid 3D sub-millimeter isotropic T1ρ mapping of the knee using motion-robust interleaved spin-lock acquisition with compressed sensing. Proc. ISMRM 2020:2690.
4. Nagawa K, et al. Accelerated high-resolution (0.5mm2) T1ρ mapping of the knee joint using interleaved spin-lock-prepared 3D gradient echo with Compressed SENSE. Proc. ISMRM 2020:3808.
5.Borthakur A, et al. T1ρ MRI of Alzheimer’s Disease. Neuroimage. 2008 Jul 15; 41(4): 1199–1205.
6. Haris M, et al. T1rho MRI and CSF biomarkers in diagnosis of Alzheimer's disease. Neuroimage Clin. 2015 Feb 26;7:598-604.
7. Pang Y. A self-compensated spin-locking scheme for quantitative R1ρ dispersion in articular cartilage. Proc. ISMRM 2020:2736.
8. Baboli R et al. Isotropic morphometry and multicomponent T1ρ mapping of human knee articular cartilage in vivo at 3T. J Magn Reson Imaging. 2018 Dec; 48(6): 1707-1716.
Figures