Hannah Mantebea1, Syeda Batool1, Mouhamad Hammami1, and Yang Xia1
1Physics, Oakland University, Rochester, MI, United States
Synopsis
This study aims to quantity the structural
morphology of sesamoid fibrocartilage in rabbits, using µMRI at 19.5-µm resolution and polarized light microscopy at 1-µm
resolution. We
show that the sesamoid fibrocartilage has one thin surface layer (~ 10 µm) of
parallel-arranged fibers followed immediately by the majority of random fibers
(~ 390 µm). T2 anisotropy was not observed in fibrocartilage. The collagen
fibers in the surface layer are dense and in parallel with the surface, which allows
it to withstand stress from the quadriceps tendon during flexion, while at the
same time to reduce pressure load from the femur.
Introduction
Knee is a complex joint that consists of tibiofemoral and patellofemoral
regions. In arthritis research using the rabbit model, the suprapatella in the
knee, an essential weight
bearing structure, was often overlooked despite its prominence and
contribution (1). This
study aims to quantity the structural morphology of sesamoid
fibrocartilage in rabbits, using µMRI
and polarized light microscopy (PLM).Methods
Sesamoid fibrocartilage from suprapatella (n=4) of 12-14 weeks-old New
Zealand rabbits were studied quantitatively. µMRI experiments used a 7Tesla/89mm Bruker Avance
IIIHD 300 NMR spectrometer. A quantitative T2 magnetization-prepared imaging sequence was
used (2), using the echo times of 2, 4, 8, 10, and 12 ms, and with a 19.5-µm
transverse resolution. Histological sections were made out of the µMRI-imaged
specimens. The PLM system was a digital
CCD camera mounted on a polarized light microscope (Leica, Germany). The output
of the camera generates two quantitative images, the optical retardation and
the angular orientation (3). Quantitative PLM images were obtained from
thin sections at 1-µm pixel
resolution.Results
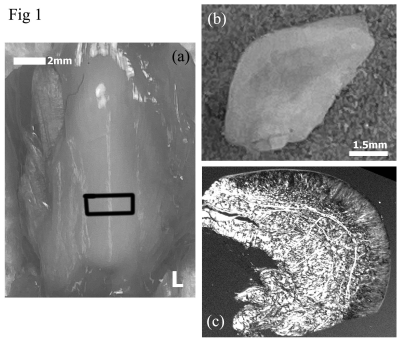
Fig 1a shows a photo of the intact posterior aspect of suprapatella that
articulates with the anterior aspect of the femur, and Fig 1b and 1c show the central-widthwise
cut surface of suprapatella, a photo (1b) and viewed under a microscope (1c). Fig
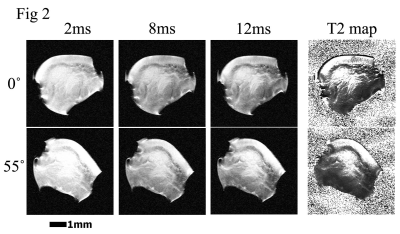
2 shows three MRI T2-weighted intensity images of a suprapatella specimen at 19.5-µm resolution, at
0˚ and 55˚ to the magnetic field (vertically up). Quantitative T2 maps of the suprapatella
(the rightest image) had a dark thin line on the surface region, which is
likely due to the magnetic susceptibility difference between tissue and air
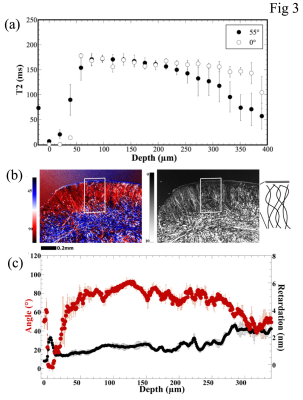
since the tissue surface bounded by air in imaging. Fig 3a shows the quantitative
1D T2 profiles from these 2D images, which lacked the magic angle effect in the
tissue (i.e., the 0˚ and 55˚ profiles were similar). Based on the T2
characteristics, the suprapatella cartilage seems to have a thin surface layer that
covers the much thicker main tissue of about 390 µm in average thickness. Both
layers in the suprapatella show little variation when the tissue blocks changed
their orientations in the magnetic field. Fig 3b shows one set of quantitative PLM
images at 1.0-µm pixel resolution. Several observations can be made from these
quantitative results. First, the top 10 µm of the sesamoid fibrocartilage can
be viewed as the surface layer (or skin) of the sesamoid fibrocartilage, which confirms
the observation in µMRI. This skin has considerable portion of the collagen
fibers being oriented in parallel with the surface of sesamoid fibrocartilage
(as in the line drawings on the furthest right of Fig 3b), which would help in
the tangential gliding when the joint is in motion. Below this organized skin, the
quantitative values in most of the fibrocartilage have large variations, in
contrast to the consistent zonal values in the PLM images for articular
cartilage (3). The retardation values for most of the non-surface fibrocartilage
varied around 1 nm, indicating the lack of a coherent fibril organizational
structure.Discussions
In contrast to the triple structural zones in articular cartilage, the
suprapatella only has two structural layers; a thin surface layer (~ 10 µm
thick) and a much thicker main tissue. The collagen fibers in the surface layer
are well organized, dense, and in parallel with the surface, which gives the surface
layer with its short T2 values (Fig 3a). This suprapatellar surface layer likely
aids to the reduction of friction when the surface slides with the femur
cartilage. The majority of the suprapatella is its main tissue body, where the
T2 values are consistently high and have no orientational dependence in the
magnetic field. The high-resolution features of the quantitative µMRI and PLM results
suggest the suprapatella has mostly the loosely packed fibers, together with
mostly free water with insignificant dipolar interaction, making it more
isotropic at the magic angle with respect to the magnetic field.Conclusion
The random arrangement of the fibers in the tissue offers flexibility to
the suprapatella cartilage and allows it to withstand stress from the
quadriceps tendon during flexion, while at the same time to reduce pressure
load from the femur. To
the best of our knowledge, this was the first quantitative imaging study that characterized
the morphological structures of sesamoid fibrocartilage in the suprapatella of
the rabbit at high resolutions. These quantitative results will be
beneficial to future studies of joint diseases using rabbits as the animal
model.Acknowledgements
Yang Xia is grateful to the National Institutes of Health (NIH) for a
R01 grant (AR 069047). The authors are in debt to Mr. Farid Badar for the
technical help in imaging, to Dr. Adam Lauver and Ms. Barbara Christian
(Department of Pharmacology & Toxicology, Michigan State University, East
Lansing, Michigan) for providing the rabbit samples.References
(1) T. Tischer, S. Milz, M. Maier, M. Schieker, and M. B. An immunohistochemical study of the
rabbit suprapatella, a sesamoid fibrocartilage in the quadriceps tendon
containing aggrecan. J. Histochem. Cytochem. 50, 955–960 (2002).
(2) Xia, Y. (1998). "Relaxation
Anisotropy in Cartilage by NMR Microscopy (µMRI) at 14 µm Resolution." Magn
Reson Med 39(6): 941-949.
(3) Xia, Y., et
al. (2001). "Quantitative In Situ Correlation Between Microscopic MRI and
Polarized Light Microscopy Studies of Articular Cartilage." Osteoarthritis
Cartilage 9(5): 393-406.