2953
Highly Efficient Single-Point Dixon-Based Fat Suppression for Ultrashort Echo Time Double Echo Steady State (UTE-DESS) Imaging of the Knee Joint1University of California, San Diego, San Diego, CA, United States, 2GE Healthcare, San Diego, CA, United States, 3Veterans Affairs San Diego Healthcare System, San Diego, CA, United States
Synopsis
In this study, we explored the feasibility of applying single-point Dixon (spDixon)-based fat suppression to a novel ultrashort echo time-based double echo steady state (UTE-DESS) sequence for highly efficient morphological musculoskeletal (MSK) imaging. Compared to the two-point Dixon-based approach used with conventional UTE-DESS MSK imaging which requires two echoes for fat-water separation, we demonstrated a new technique which requires only a single image in, removing the need for additional data acquisitions. The feasibility and efficacy of our approach were demonstrated in human knee joints.
Introduction
In double echo steady state (DESS) imaging, fat signal commonly appears with high intensity due to a high T2/T1 ratio. To suppress this signal in DESS, the water excitation technique using a spectral-spatial radiofrequency (RF) pulse has been widely used1–6. Recently, an ultrashort echo time-based DESS (UTE-DESS) sequence was deemed feasible for imaging both short and long T2 tissues in human knee joint7. UTE-DESS imaging is disadvantaged by strong fat signal (just as in DESS), but unfortunately the conventional water excitation technique using composite, long-duration (~10ms) RF pulses8,9 cannot be applied in UTE-DESS targeting TEs shorter than 0.1ms. Alternatively, fat suppression can be achieved with two-point Dixon7, though this approach requires a longer scan time to acquire an additional set of images with a different time delay. In this study, we showed the feasibility of efficient single-point Dixon (spDixon)-based fat suppression in UTE-DESS for morphological musculoskeletal imaging.Methods
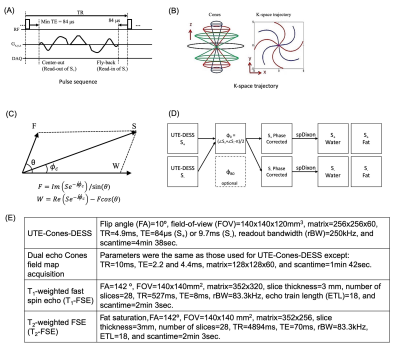
Figures 1A and 1B show the pulse sequence diagram and k-space trajectory of 3D Cones-based UTE-DESS (UTE-Cones-DESS) sequence used in this study to acquire two images from free induction decay (FID)-like S+ and echo-like S- signal. The spDixon method decomposes complex MR signal into individual fat and water signals with a known chemical shift-induced phase difference, θ (Figure 1C)10,11. A prerequisite of spDixon is the removal of any phase errors which have been added to the complex MR signal, including initial phase offset, $$$\phi_{0}$$$, and phase error due to B0 field inhomogeneity, $$$\phi_{B0}$$$. In general, $$$\phi_{B0}$$$ is determined by the field inhomogeneity, ΔB0, and by a time delay during FID, $$$\tau$$$, such that $$\phi_{B0} = \gamma ΔB_0 \tau$$, where $$$\gamma$$$ is the gyromagnetic ratio of proton. $$$\phi_{0}$$$ is more complicated to model as it involves many factors including electric property of tissues, RF coil, data readout, and reconstruction. Here, we proposed to use the intrinsic signal properties of S+ and S- in DESS to measure $$$\phi_{0}$$$ without any additional acquisitions. S- is acquired at an echo time (TE) of (2xTR–$$$\tau$$$) in the signal passage toward refocusing in the opposite direction to S+ on the transverse plane. Therefore, the phase of S+ and S- in DESS can be written as $$\phi_{S_+} = \phi_{0}+\phi_{B0}+\phi_{c},$$ $$\phi_{S_-} = \phi_{0}-\phi_{B0}-\phi_{c}+\pi,$$ where $$$\phi_{c}$$$ is the phase of the complex signal resulting from a combination of fat and water signals. Then, $$$\phi_{0}$$$ can be solved for by using both equations such that $$\phi_{0} = (\phi_{S_+}+\phi_{S_-}-\pi)/2.$$Figure 1D shows a block diagram for the proposed workflow to perform spDixon in UTE-DESS imaging, where it is expected that $$$\phi_{B0}$$$ will be much smaller than $$$\phi_{0}$$$ because of the short $$$\tau$$$. Therefore, correction of $$$\phi_{B0}$$$ is merely optional for rapid morphological UTE-DESS imaging. To evaluate the feasibility, six healthy volunteers (two females, aged 35.3±8.8 years) and five patients with osteoarthritis (OA) (five males, aged 51.0±8.1 years) were recruited and underwent knee imaging in a 3T MRI scanner (GE-MR750) using a transmit/receive 8-channel knee coil. The protocol included UTE-Cones-DESS, field map acquisition, T1-weighted fast spin echo (T1-FSE), and T2-weighted fast spin echo (T2-FSE), as shown in Figure 1E.
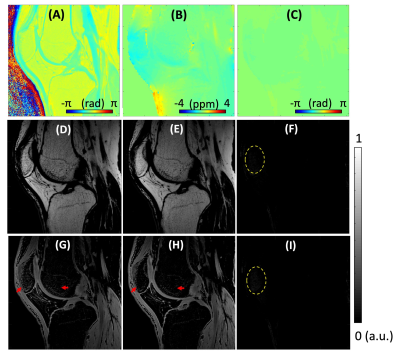
Results
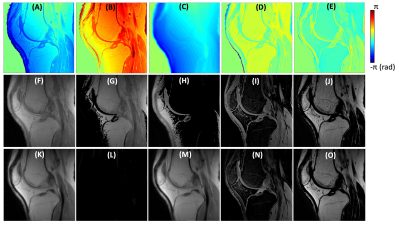
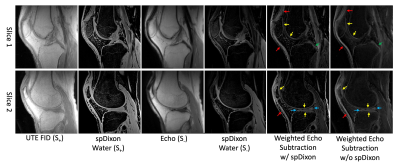
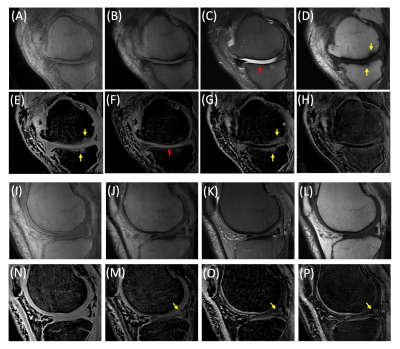
Figure 2 shows the results of spDixon in UTE-DESS (37-year-old male). Without correction of $$$\phi_{0}$$$, spDixon yielded largely misestimated water and fat signals (Figures 2G,H,L,M), while with correction of $$$\phi_{0}$$$ spDixon provided more reasonable fat and water images (Figures 2I,J,N,O). The estimated $$$\phi_{B0}$$$ for the prescribed $$$\tau$$$ was negligibly small (Figure 3C) compared to $$$\phi_{c}$$$ (Figure 3A) and $$$\phi_{0}$$$ (Figure 2C). Figures 3D-I show the comparison between spDixon without and with correction of $$$\phi_{B0}$$$: the difference was small and did not impair morphological information (red arrows). Figure 4 shows the results of UTE-DESS imaging with spDixon on the same volunteer, in which fat-suppressed water images were obtained for both S+ and S- by utilizing the proposed spDixon approach. Both weighted echo-subtraction (WES) with and without spDixon achieved high contrast specific to the short T2 tissues for the osteochondral junction (yellow arrows), tendons (red arrows), menisci (blue arrows), and ligaments (green arrows).Figure 5A-H shows results from a representative OA patient (52-year-old male). Long T2 water signal from fluid is well-detected in the water image of S-, which corresponds well with the T2-FSE image (red arrows in Figures 5C,F). Sclerosis of subchondral bone is also well-depicted as bright contrast in the S+ water image and the WES water image (yellow arrows in Figures 5D,E,G). Note that the lesion is not well-depicted in the WES image without spDixon due to the unsuppressed fat signal (Figure 5H). Figures 5I-P show results from another OA patient (56-year-old male). Meniscal tear is detected in the S- water image and the WES water image with or without spDixon (yellow arrows in Figure 5M-P), which corresponds well with the T2-FSE and T1-FSE images.
Discussion and Conclusion
We demonstrated that fat suppression overall improved lesion detection of long T2 water and subchondral bone sclerosis (Figure 5). spDixon-based fat suppression strategy has the advantage of shorter scan time over the two-point Dixon-based approach since intrinsic information derived from the UTE-DESS signal is utilized. The proposed UTE-DESS with spDixon may provide a new imaging tool to assess short T2 tissues such as the osteochondral junction, tendons, ligaments, and menisci.Acknowledgements
The authors acknowledge grant support from the NIH (R01AR062581, R01AR068987, R01AR075825, and R21AR075851), Veterans Affairs (I01RX002604 and I01CX001388), and GE Healthcare.References
1. Kohl S, Meier S, Ahmad SS, et al. Accuracy of cartilage-specific 3-Tesla 3D-DESS magnetic resonance imaging in the diagnosis of chondral lesions: comparison with knee arthroscopy. J. Orthop. Surg. Res. 2015;10:191 doi: 10.1186/s13018-015-0326-1.
2. Friedrich KM, Reiter G, Kaiser B, et al. High-resolution cartilage imaging of the knee at 3T: Basic evaluation of modern isotropic 3D MR-sequences. Eur. J. Radiol. 2011;78:398–405 doi: 10.1016/j.ejrad.2010.01.008.
3. Moriya S, Miki Y, Yokobayashi T, Ishikawa M. Three-Dimensional Double-Echo Steady-State (3D-DESS) magnetic resonance imaging of the knee: Contrast optimization by adjusting flip angle. Acta radiol. 2009;50:507–511 doi: 10.1080/02841850902849444.
4. Moriya S, Miki Y, Matsuno Y, Okada M. Three-dimensional double-echo steady-state (3D-DESS) magnetic resonance imaging of the knee: establishment of flip angles for evaluation of cartilage at 1.5 T and 3.0 T. Acta radiol. 2012;53:790–794 doi: 10.1258/ar.2012.110532.
5. Fujii H, Fujita A, Yang A, et al. Visualization of the peripheral branches of the Mandibular division of the trigeminal nerve on 3D double-echo steady-state with water excitation sequence. Am. J. Neuroradiol. 2015;36:1333–1337 doi: 10.3174/ajnr.A4288.
6. Qin Y, Zhang J, Li P, Wang Y. 3D Double-Echo Steady-State with Water Excitation MR Imaging of the Intraparotid Facial Nerve at 1.5T: A Pilot Study. Am. J. Neuroradiol. 2011;32:1167–1172 doi: 10.3174/ajnr.A2480.
7. Chaudhari AS, Sveinsson B, Moran CJ, et al. Imaging and T2 relaxometry of short-T2 connective tissues in the knee using ultrashort echo-time double-echo steady-state (UTEDESS). Magn. Reson. Med. 2017;78:2136–2148 doi: 10.1002/mrm.26577.
8. Hardy PA, Recht MP, Piraino DW. Fat suppressed MRI of articular cartilage with a spatial-spectral excitation pulse. J. Magn. Reson. Imaging 1998;8:1279–1287 doi: 10.1002/jmri.1880080615.
9. Schick F, Forster J, Machann J, Huppert P, Claussen CD. Highly selective water and fat imaging applying multislice sequences without sensitivity toB1 field inhomogeneities. Magn. Reson. Med. 1997;38:269–274 doi: 10.1002/mrm.1910380216.
10. Ma J. A single-point dixon technique for fat-suppressed fast 3D gradient-echo imaging with a flexible echo time. J. Magn. Reson. Imaging 2008;27:881–890 doi: 10.1002/jmri.21281.
11. Jang H, Carl M, Ma Y, et al. Fat suppression for ultrashort echo time imaging using a single-point Dixon method. NMR Biomed. 2019:e4069 doi: 10.1002/nbm.4069.
Figures