2951
The value of magnetic resonance T2*mapping for early detection of knee cartilage damage in hemophilia arthropathy1Fuwai Central China Cardiovascular Hospital, Zhengzhou, China, 2MR Collaboration, Siemens Healthcare Ltd., Beijing, China, Beijing, China, 3Siemens Shenzhen Magnetic Resonance Ltd, Shenzhen, China
Synopsis
Early detection and therapy of articular cartilage damage are key to the management of hemophilia arthropathy (HA). Magnetic resonance (MR) T2* mapping has been used to detect iron deposits in the brain and liver but rarely in HA. We compared T2*mapping results of 15 HA patients (18 knees) and 10 volunteers (18 knees). We found that the average T2* values of the medial femoral condyle and medial tibial plateau cartilage in HA patients were significantly lower than in the normal group, suggesting the importance of T2* mapping in the quantitative assessment of iron deposition for early cartilage damage diagnosis in HA patients.
Purpose
Repeated joint bleeding in hemophilia patients leads to joint cartilage damage and even joint dysfunction, which is the main cause of joint damage and disability in hemophilia patients. Prevention therapy is an effective way to avoid the occurrence of hemophilia osteoarthropathy, and the early diagnosis and monitoring of joint changes help improve prognosis. The main purpose of imaging examinations for hemophilia is to detect early changes to evaluate the effectiveness of treatment options and select an appropriate preventive treatment strategy. Conventional sequences such as T1WI, T2WI, and PDWI can demonstrate joint disease such as synovial thickening, joint cavity effusion/hemorrhage, and subchondral cyst degeneration but cannot provide quantitative indicators and are not sensitive enough to detect early changes in cartilage disease. T2* mapping has been extensively used in the early diagnosis of brain and liver diseases [1-2] but rarely to detect knee cartilage damage in patients with hemophilia. Therefore, the purpose of this study was to explore the value of magnetic resonance T2* mapping in detecting early knee cartilage damage in patients with hemophilia.Methods
This study prospectively recruited fifteen male patients (age: 18.6 ± 6.1 years, 18 knees) with confirmed hemophilia joint disease from the Hemophilia Diagnosis and Management Center in Henan Province, China. Ten age- and gender-matched volunteers (age: 20.8 ± 5.5 years, 18 knees) were recruited as healthy controls (HC) from the local community. All the participants were scanned on a 3T MAGNETOM Skyra scanner (Siemens Healthcare, Erlangen, Germany) equipped with a 15-channel knee coil using conventional sequences including T1WI, PDWI, and a T2* mapping sequence. The parameters for the T2* mapping sequence were as follows: TR = 890 ms; TE = 4.36/11.90/19.44/26.98/34.52 ms for five echoes; FOV = 160 x 160 mm2; voxel size = 0.4 x 0.4 x 4.0 mm3; acquisition time = 6:50 mins. The joints were scored as levels 0 – 4, according to the International Prophylaxis Study Group (IPSG). Because articular cartilage damage with a score greater than 2 was too severe to be analyzed quantitatively, this group provided little value for our early damage detection research and was excluded. Finally, 18 cases with cartilage loss items less than or equal to 2 points were enrolled in this study. The T2* mapping data were analyzed using manually drawn regions of interest (ROIs) on the syngo.via workstation (Siemens Healthcare). The ROIs included 5 cartilage units, including the patella, lateral and medial femoral condyle, and lateral and medial tibial condyle. The measurement was repeated three times to acquire the average T2* value for each. The T2* value was compared between the two groups for each ROI with independent samples t-test using SPSS 22.0 software (IBM SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.Results
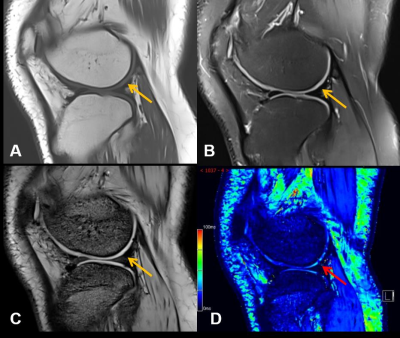
Figure 1 shows that MR T2* mapping could detect some additional cartilage damages compared to conventional MRI. As shown in Figure 2 the HA patients had significantly lower T2* values than the control group in the medial femoral condyle (28.8 ± 3.4 vs. 31.5 ± 3.6 ms, P=0.027) and medial tibial plateau (21.9 ±3.8 vs. 23.7 ± 4.0, P=0.012). No significant difference was found between these two groups in other ROIs.Discussion
This study found that the T2* value of knee cartilage in patients with hemophilia was lower than that of the healthy group. Previous studies that used T2* mapping to investigate osteoarthritis found an increase of T2* value in the patients’ knees. This is perhaps due to the distinct HA pathogenesis of repeated bleeding. Paramagnetic substances can reduce the T2* value by affecting the uniformity of the local magnetic field, such as deoxyhemoglobin, methemoglobin, or hemosiderin in lesions and tissues[3]. In the affected joints of patients with HA, the cleansing capacity of the synovium may be overloaded with continuous or repeated bleeding, causing iron to accumulate in the form of hemosiderin[4], thus reducing the T2* value. One study also confirmed iron deposition within chondrocytes using histological analysis of cartilage, associated with decreased T2* values. Iron can be deposited not only in the synovium and joint cavity, but also in cartilage, and cartilage iron can be used as a new biomarker[5]. This indicates that the T2* mapping sequence can quantitatively detect the amount of iron deposition in the cartilage of HA patients for early detection of cartilage damage, which is helpful for the management of HA.Conclusion
MR T2* mapping is useful for the quantitative assessment of iron deposition in knee cartilage, which is helpful for early diagnosis of cartilage damage and the management of HA patients.Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 81271543).References
[1] Padeniya P, Siriwardana S, Ediriweera D et al. Comparison of liver MRI R2(FerriScan®)VS liver MRI T2* as a measure of body iron load in a cohort of beta thalassaemia major patients[J]. Orphanet Journal of Rare Diseases, 2020, 15.
[2] Tambasco N, PaoliniPaoletti F, Chiappiniello A, et al. T2*-weighted MRI values correlate with motor and cognitive dysfunction in Parkinson's disease [J]. Neurobiol Aging, 2019, 80(91-98).
[3] Van Vulpen LFD, Holstein K, Martinoli C. Joint disease in haemophilia: Pathophysiology, pain and imaging [J]. Haemophilia, 2018, 24:44-49.
[4] Chavhan GB, Babyn PS, Thomas B, et al. Principles, techniques, and applications of T2*-based MR imaging and its special applications [J]. Radiographics, 2009, 29(5): 1433-1449.
[5] Von Drygalski A, Barnes RFW, Jang H, et al. Advanced magnetic resonance imaging of cartilage components in haemophilic joints reveals that cartilage hemosiderin correlates with joint deterioration [J]. Haemophilia, 2019, 25(5): 851-858.
Figures
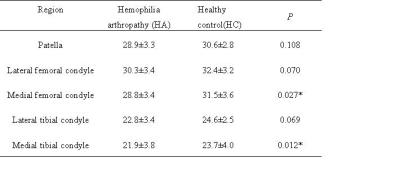
T2* values of articular cartilage in different regions (mean ± SD)
Note: * P<0.05 indicates statistical significance