2944
Direct Mapping of the Nucleus Accumbens Core and Shell using Deep Brain Stimulation with functional Magnetic Resonance Imaging in rats1Center for Animal MRI, Biomedical Research Imaging Center, University of North Carolina, Chapel Hill, NC, United States, 2Actuated medical, Bellefonte, PA, United States
Synopsis
Nucleus accumbens (NAc) is known as the backbone of the reward circuit, which the core and shell appear to be dominant. To directly map their distinct functional connectivity structure, we employed simultaneous Deep Brain Stimulation (DBS) at NAc during functional Magnetic Resonance Imaging (fMRI) and demonstrated the difference of the functional organization between core and shell. MR compatible 16ch microprobe was utilized to stimulate multiple regions during single scan session. We expect this technique could be useful to dissect the therapeutic circuit of DBS in NAc in the preclinical approach.
INTRODUCTION
The nucleus accumbens (NAc) has a significant role in the cognitive processing of motivation, reward and reinforcement.1–3 Also, it has a significant role in addiction.4 The NAc consists of shell and core and each is known to have a distinct role, which established through behavior test5 and slice recording6, however, the distinct functional circuits have not been investigated systemically in rats. Functional Magnetic Resonance Imaging (fMRI) allows researchers to detect various degrees of activity throughout the brain by analyzing blood oxygen level-dependent (BOLD) activity, so it is a useful tool for seeing the whole brain network systemically. Deep brain stimulation of the nucleus accumbens (NAc-DBS) is an emerging therapy for diverse, refractory neuropsychiatric diseases.7,8 Although DBS therapy is broadly hypothesized to work through large-scale neural modulation, little is known regarding the neural circuits and networks affected by NAc-DBS cause it is very hard to do in vivo. Using DBS-fMRI can provide a state-of-the-art comprehensive understanding of the neural networks impacted by DBS in vivo. In this study, we use the MR-compatible 16-channel microelectrode for electric DBS, which capable to stimulate multiple regions with single probe implantation. Such advantages allowed us to dissect the functional circuit of core and shell of NAc in a preciseness and selective manner during fMRI experiments.METHODS
A total of 9 male Sprague-Dawley rats (250-400g, Charles River Laboratories, Wilmington MA) were used. For electrical DBS experiments, microelectrode was implanted to the mediodorsal boundary of the NAc core/shell at 1.5 mm anterior to bregma, 2 mm right of midline, and 7.5 mm ventral to the cortical surface (Figure 1A). The T2-weighted anatomical image was acquired using a fast low angle shot (FLASH) sequence prior to the fMRI scan session to evaluate the implanted location of the electrode. (Scan parameters: TR = 2700 ms, TE = 10 ms, FlipAngle = 40°, bandwidth = 250 kHz, slice thickness = 0.2 mm, number of slices = 64, matrix size = 144 x 144 and FOV = 2.88× 2.88 cm2). Blood-Oxygen-Level-Dependent (BOLD)-fMRI scans were acquired using a multi-slice single-shot gradient echo echo-planar imaging sequence (GE-EPI) (Scan parameters: TR = 2000 ms, TE = 14 ms, bandwidth = 250 kHz, slice thickness = 0.4 mm, number of slices = 32, matrix size = 72 × 72 and FOV = 2.88× 2.88 cm2). Each stimulation period consisted of a series of charge-balanced square-wave pulses delivered at 65 Hz, with a stimulation intensity of 200 μA and a pulse duration of 10 s. A 420 s block design paradigm was implemented, consisting of 120 s of rest (stimulation OFF) followed by 20 s of stimulation ON and an additional 120 s of rest (stimulation OFF). These three stimuli were given to channels 1, 8, and 16 respectively (Figure 1B), and repeated 5 times per one animal.RESULTS
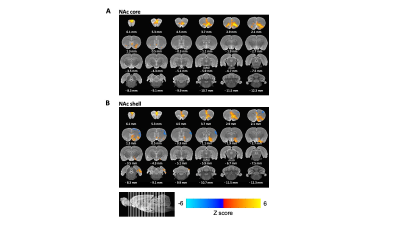
The electrode channels are positioned to target both the core and shell of NAc. In most cases, channel 1 was positioned at the NAc shell and channel 16 was positioned at the NAc core. The DBS-evoked fMRI results indicated that the stimulation of the shell appears to increase BOLD response in the unilateral medial prefrontal cortex (mPFC), anterior cingulate, and amygdala, which is similar to the previous study7, whereas the stimulation of the core increases the BOLD response at bilateral mPFC and the orbitofrontal cortex area.DISCUSSION and CONCLUSION
In this study, we performed multimodal fMRI procedures to identify neural circuitry modulated by NAc-DBS in a healthy rat model. It is well-known that NAc is an important component of the reward system. 9,10 The shell of NAc has been identified to mediate the reinforcing properties of rewarding substances11. Our fMRI result demonstrated that the shell has a strong functional connection with the anterior cingulate and amygdala which are the major reward-hub. 11 Behavior studies also support that shell is mainly related to reward-behavior.12 Evoked fMRI with electrical DBS uncovers a broad range of cortical and subcortical regions displaying stimulation-induced BOLD increases, including the prefrontal cortex, amygdala, or other regions. Collectively, our results are expected to be a useful approach to illuminating how DBS exerts therapeutic effects on the entire brain function network.Acknowledgements
We thank the Center for Animal MRI (CAMRI) at the University of North Carolina at Chapel Hill for their helpful support valuable discussions concerning the experiments described in this manuscript. This study was supported by NIH (Grant No: R42DA051265, RF1MH117053, and R01 MH111429)References
1. Mogenson, G. J., Jones, D. L. & Yim, C. Y. From motivation to action: Functional interface between the limbic system and the motor system. Prog. Neurobiol. 14, 69–97 (1980).
2. Soares-Cunha, C. et al. Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol. Psychiatry 3241–3255 (2019). doi:10.1038/s41380-019-0484-3
3. Wenzel, J. M., Rauscher, N. A., Cheer, J. F. & Oleson, E. B. A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature. ACS Chem. Neurosci. 6, 16–26 (2015).
4. Calipari, E. S. et al. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc. Natl. Acad. Sci. U. S. A. 113, 2726–2731 (2016).
5. Jones, S. R., O’Dell, S. J., Marshall, J. F. & Wightman, R. M. Functional and anatomical evidence for different dopamine dynamics in the core and shell of the nucleus accumbens in slices of rat brain. Synapse 23, 224–231 (1996).
6. Drew, P. J. Vascular and neural basis of the BOLD signal. Curr. Opin. Neurobiol. 58, 61–69 (2019).
7. Lai, H. Y., Younce, J. R., Albaugh, D. L., Kao, Y. C. J. & Shih, Y. Y. I. Functional MRI reveals frequency-dependent responses during deep brain stimulation at the subthalamic nucleus or internal globus pallidus. Neuroimage 84, 11 (2014).
8. Min, H. K. et al. Deep brain stimulation induces BOLD activation in motor and non-motor networks: An fMRI comparison study of STN and EN/GPi DBS in large animals. Neuroimage 63, 1408–1420 (2012).
9. Stuber, G. D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–382 (2011).
10. Nicola, S. M. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl). 191, 521–550 (2007).
11. Bossert, J. M., Poles, G. C., Wihbey, K. A., Koya, E. & Shaham, Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J. Neurosci. 27, 12655–12663 (2007).
12. Sesia, T. et al. Deep brain stimulation of the nucleus accumbens core and shell: Opposite effects on impulsive action. Exp. Neurol. 214, 135–139 (2008).
Figures