2943
The role of central vestibular system in circadian rhythms?
Alex T. L. Leong1,2, Christopher Man1,2, Yong Gu3, and Ed X. Wu1,2
1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China, 3Institute of Neuroscience, CAS Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China
1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China, 3Institute of Neuroscience, CAS Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China
Synopsis
The vestibular system is critical for bodily functions, yet it remains an underappreciated sense. Recently, seminal studies of the prolonged exposure to microgravity in space showed significant alteration in vestibular activity and circadian rhythms. However, little is known regarding the role of the central vestibular system in regulating the circadian clock. Here, we deploy fMRI and optogenetic stimulation of vestibular excitatory neurons to visualize the neural targets of the vestibular system in circadian circuits. We reveal that the central vestibular system provides inputs to the suprachiasmatic nucleus, optic nerve and locus coeruleus, notable regions that drive and regulate circadian rhythms.
Purpose
Among our sensory systems, five senses - sight, hearing, touch, smell and taste - receive the most attention leaving our vestibular sense as the least understood. The vestibular system senses angular and linear acceleration of the head in three dimensions via a labyrinth of sense organs in the inner ear, supporting numerous functions from gaze stabilization and postural control, to high-level cortical functions involving spatial cognition, navigation and memory1-4. Recently, studies revealed that vestibular activities also act as vital inputs for synchronizing the circadian rhythms in animals and in humans5,6. Lesions to the vestibular sense organs were shown to alter the daily sleep-wake cycle and sleep quality7, while sleep deprivation led to temporarily, and in some cases permanently, impaired motor functions related to postural and balance control8. At present, it remains a challenge to visualize the brain-wide central vestibular pathways that mediate such a multitude of critical functions. In this study, we will deploy an optogenetic functional MRI (fMRI) approach that can stimulate the vestibular nucleus (VN) excitatory neurons and examine the spatially segregated yet functionally integrated vestibular functions. Specifically, we sought to examine whether regions that are responsible for the control of circadian rhythm (i.e., sleep-wake cycle) are targeted by one of the four major vestibular nuclei, the medial VN (MVN), across multi-synaptic pathways throughout the brain.Methods
Animal preparation and optogenetic stimulation: 3μl of AAV5-CaMKIIa::ChR2(H134R)-mCherry was injected to MVN (-11.5mm posterior to Bregma, +1.5mm medial-lateral right hemisphere, -8.5mm from surface of dura) of adult SD rats (200-250g, male, n=18; Figure 1A). Four weeks after injection, an opaque optical fiber cannula (d=450μm) was implanted at the injection site. Blue (473nm) light was presented to animals expressing ChR2 at 20Hz (20% duty cycle, 40mW/mm2) in a block-design paradigm (20s on and 60s off; Figure 1B).fMRI acquisition and analysis: fMRI data were acquired on 7T Bruker scanner using GE-EPI (FOV=32×32mm2; matrix=64×64; α=56°; TE/TR=20/1000ms; and 16 contiguous slices with 1mm thickness). Data were preprocessed before standard GLM analysis was applied to identify significant BOLD responses (P<0.05).
Results
Brain-wide fMRI reveals downstream signal propagation from MVN to circadian circuits: We found BOLD fMRI activations in regions that have been linked extensively to the generation and maintenance of the circadian clock. These regions include the suprachiasmatic nucleus (SCN), arcuate nucleus (ARC), the posterior and lateral part of the hypothalamus, and locus coeruleus (LC). Previously, we reported robust large-scale activations at numerous cortical, hippocampal formation and subcortical regions9 (Figure 2). Notable regions that were activated include sensorimotor cortices and their associated thalamus (auditory, visual, somatosensory and motor), high order cortices involved in cognition (cingulate, retrosplenial, temporal association and parietal), and the hippocampal formation involved in spatial navigation (dentate gyrus, entorhinal cortex and subiculum). Furthermore, we found broad activations at the midbrain which has extensive projections to thalamic and hippocampal formation regions (mammillary nucleus and periaqueductal gray). As expected, the oculomotor nucleus, an essential midbrain region mediating the vestibulo-ocular reflex, was also activated. Coupled with the previous reported large-scale activations, our present findings indicate that the circadian rhythms are likely significantly affected by vestibular inputs.Discussion
The vestibular system vastly differs from other primary sensory systems, such as auditory, visual and somatosensory systems. It encompasses a more complex organization of its pathways with overlapping inputs from sense organs and other sensory thalamic nuclei to individual vestibular nuclei and numerous efferent projections from the vestibular nuclei across hemispheres to sensory/non-sensory midbrain regions and the hippocampal formation1. Moreover, unlike primary sensory processing, central vestibular processing is distributed across multiple regions10. Given these complexities and challenges, the role of the vestibular system in circadian rhythms and its broad neural targets remain poorly understood. Here, we found activations at SCN, which is a principal circadian clock that is entrained to the daily light- and dark-cycle11. This indicates that SCN also receives inputs from the central vestibular system, in addition to the known direct retinal innervation, which could affect the body’s circadian rhythms. Notably, we also found activations in the optic nerve (OPT), suggesting that vestibular activity can also directly affect inputs from the retina before they reach the SCN. Circadian rhythms are also known to be affected by the endocrine system, which regulates our general arousal, appetite and metabolic levels12. We observed activations at the posterior and lateral hypothalamus, and LC, indicating that vestibular inputs do play a role in regulating the release of neurotransmitter and hormones to maintain a healthy circadian clock. Our findings provide new insight into the role of vestibular activity in the regulation of circadian rhythms.Conclusion
In summary, we revealed notable regions that are pivotal for the functions of circadian circuits activated by the stimulation of the vestibular nucleus, the nexus of vestibular processing in the central nervous system. This study presents an integrative fMRI platform to gain critical knowledge on the role of the vestibular system in circadian rhythms.Acknowledgements
This study was supported by Hong Kong Research Grant Council (R7003-19, C7048-16G, HKU17112120, HKU17103819 and HKU17104020), Guangdong Key Technologies for Treatment of Brain Disorders (2018B030332001), and Guangdong Key Technologies for Alzheimer's Disease Diagnosis and Treatment (2018B030336001).References
- Vidal, P.P., Cullen, K., Curthoys, I.S., Du Lac, S., Holstein, G., Idoux, E., Lysakowski, A., Peusner, K., Sans, A. & Smith, P. The Vestibular System. in The Rat Nervous System 805-864 (Academic Press, San Diego, 2015).
- Goldberg, J.M. Vestibular Inputs: The Vestibular System. in Neuroscience in the 21st Century (ed. Pfaff, D.W.) 883-929 (Springer New York, New York, NY, 2013).
- Hitier, M., Besnard, S. & Smith, P.F. Vestibular pathways involved in cognition. Frontiers in integrative neuroscience 8, 59 (2014).
- Cullen, K.E. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci 35, 185-196 (2012).
- Besnard, S., Tighilet, B., Chabbert, C., Hitier, M., Toulouse, J., Le Gall, A., Machado, M.L. & Smith, P.F. The balance of sleep: Role of the vestibular sensory system. Sleep Med Rev 42, 220-228 (2018).
- Kompotis, K., Hubbard, J., Emmenegger, Y., Perrault, A., Muhlethaler, M., Schwartz, S., Bayer, L. & Franken, P. Rocking Promotes Sleep in Mice through Rhythmic Stimulation of the Vestibular System. Curr Biol 29, 392-401 e394 (2019).
- Martin, T., Mauvieux, B., Bulla, J., Quarck, G., Davenne, D., Denise, P., Philoxene, B. & Besnard, S. Vestibular loss disrupts daily rhythm in rats. J Appl Physiol (1985) 118, 310-318 (2015).
- Zhang, J., Li, B., Yu, L., He, Y.C., Li, H.Z., Zhu, J.N. & Wang, J.J. A role for orexin in central vestibular motor control. Neuron 69, 793-804 (2011).
- Leong, A.T.L., Gu, Y., Chan, Y.S., Zheng, H., Dong, C.M., Chan, R.W., Wang, X., Liu, Y., Tan, L.H. & Wu, E.X. Optogenetic fMRI interrogation of brain-wide central vestibular pathways. Proc Natl Acad Sci U S A 116, 10122-10129 (2019).
- Fattal, D., Hansen, M. & Fritzsch, B. Aging-Related Balance Impairment and Hearing Loss. in The Wiley Handbook on the Aging Mind and Brain 315-336 (2018).
- Hastings, M.H., Maywood, E.S. & Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci 19, 453-469 (2018).
- Bedrosian, T.A., Fonken, L.K. & Nelson, R.J. Endocrine Effects of Circadian Disruption. Annu Rev Physiol 78, 109-131 (2016).
Figures
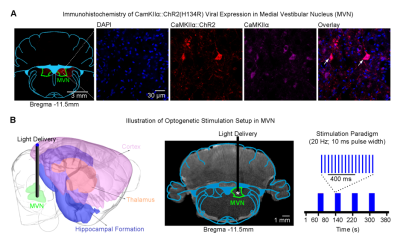
Figure 1. Illustration of CaMKIIa::ChR2 viral expression in medial vestibular nucleus (MVN) excitatory neurons and optogenetic fMRI stimulation setup. (A) ChR2-mCherry expression in MVN. Lower-magnification (left) and higher-magnification (right). Overlay of images revealed colocalization of mCherry and CaMKII in the cell body of MVN neurons (indicated by white arrows). (B) Illustration of the stimulation site in ChR2 MVN excitatory neurons during optogenetic fMRI experiments (asterisk, stimulation site).
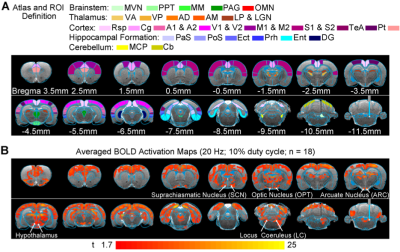
Figure 2. Brain-wide fMRI revealed activations at regions mediating circadian rhythms upon optogenetic stimulation of MVN excitatory neurons. (A) Illustration of Paxinos atlas-based ROI definitions in the vestibular and midbrain, thalamic, cortical, and hippocampal formation regions. (B) Averaged BOLD activation maps at 20Hz optogenetic stimulation (n=18; P<0.05; asterisk: stimulation site). Notably, these activations include SCN, OPT, ARC, hypothalamus and LC, regions that are known to drive and regulate the circadian clock.