2942
Brain-wide functional organization of the central vestibular pathways: an optogenetic fMRI study1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China, 3Department of Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Synopsis
The vestibular system is essential to our sense of balance and spatial orientation. fMRI mapping of the vestibular system has been challenging due to the physical constraints limiting a subject’s ability to perform motion, balance, and orientation related tasks within an MRI scanner. As such, our present knowledge of the brain-wide cortical and subcortical regions that participate in processing the vestibular sense is scarce. Here, we combined fMRI and optogenetic stimulation of vestibular excitatory neurons to visualize two distinct brain-wide functional organization of central vestibular pathways originating from two major vestibular subnuclei, the superior vestibular nucleus and medial vestibular nucleus.
Purpose
The vestibular system is critical for our body functions, including maintaining body balance, coordinating body movements, and stabilizing our vision while sensing the speed, direction, and spatial orientation when the head is moving1-4. The vestibular nucleus (VN) is located in the brainstem and consists of four subnuclei1,2, superior VN (SVN), medial VN (MVN), lateral VN (LVN), and inferior VN (IVN). Early anatomical tracer studies in animal models showed extensive axonal projects coursing in and out of individual vestibular subnuclei5-8. These studies also indicated that the vestibular system plays a role in cross-modal sensory processing and cognition2,3,9. At present, despite the extensive knowledge of the broad range of anatomical projections and targets in both brain hemispheres for each vestibular subnuclei, where and how vestibular information is distributed, localized, and lateralized in the brain remain unresolved. Recently, the combined use of optogenetic and large field-of-view fMRI has enabled us to perturb long-range networks through focal, cell-type specific neural stimulation; and simultaneously monitor the brain-wide neural activities evoked by such optogenetic perturbation10,11. Taking advantage of this capability, we examine the brain-wide projections of the central vestibular pathways. Here, we aim to map, categorize and characterized the functional organizations of two major vestibular subnuclei: SVN and MVN by stimulating their respective excitatory neurons.Methods
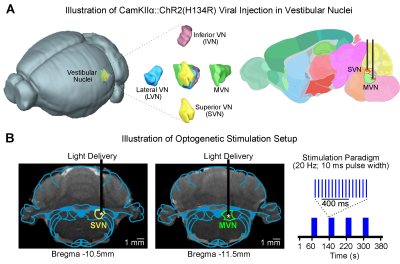
Animal preparation and optogenetic stimulation: 3μl of AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to SVN (-10.5mm posterior to Bregma, +2.2mm medial-lateral right hemisphere, -7.2mm from surface of dura) and MVN (-11.5mm posterior to Bregma, +1.5mm medial-lateral right hemisphere, -8.5mm from surface of dura) of adult rats (200-250g, male, SD strain, SVN n=8 & MVN: n=18). Four weeks after injection, an opaque optical fiber cannula (d=450μm) was implanted at the injection site (Figure 1A, B). Blue (473nm) light was presented to animals expressing ChR2 at 20Hz (20% duty cycle, 40mW/mm2) in a block-design paradigm (20s on and 60s off; Figure 1B).fMRI acquisition and analysis: fMRI data was acquired on 7T Bruker scanner using GE-EPI (FOV=32×32mm2, matrix=64×64, α=56°, TE/TR=20/1000ms, and 16 contiguous slices with 1mm thickness). Data were preprocessed before standard GLM analysis was applied to identify significant BOLD responses (p<0.01; FDR corrected).
Results
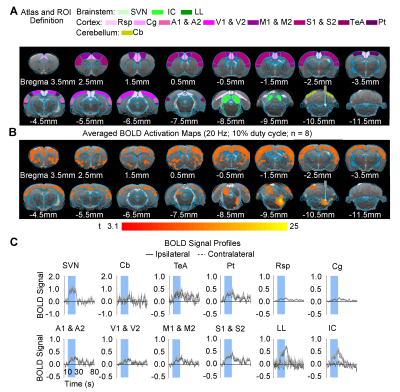
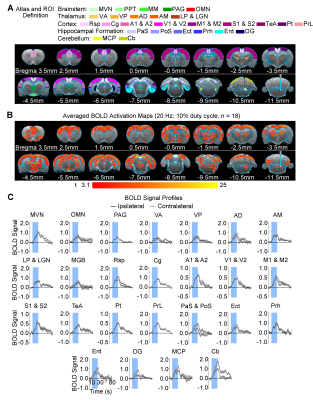
Brain-wide fMRI mapping of downstream signal propagation from SVN: We detected robust BOLD fMRI activations at numerous cortical and subcortical regions including cerebellum and brainstem (Figure 2). Notable regions that were activated include the sensorimotor cortices (auditory, visual, somatosensory, and motor), high order cortices involved in cognition (cingulate, retrosplenial, temporal association and parietal), cerebellar cortex and auditory-related brainstem structures. Interestingly, we found unilateral activations in the auditory midbrain structures, including contralateral inferior colliculus and ipsilateral lateral lemniscus.Brain-wide fMRI mapping of downstream signal propagation from MVN: Brain-wide BOLD fMRI activations at numerous cortical, hippocampal formation, and subcortical regions were identified (Figure 3). Similar to SVN results, notable activations were found in sensorimotor cortices and high order cortices involved in cognition, as well as cerebellar cortex. Furthermore, MVN excitation also evoked BOLD activations in the hippocampal formation (dentate gyrus, entorhinal cortex, and subiculum) and the midbrain structures which project to thalamic and hippocampal formation regions (mammillary nucleus and periaqueductal gray). Moreover, the oculomotor nucleus, an essential midbrain region mediating the vestibulo-ocular reflex, was also activated.
Categorization of distinct vestibular pathways recruited by vestibular subnuclei: Numerous vestibular pathways were identified by the optogenetic excitation of two neighboring vestibular subnuclei (i.e., SVN and MVN). Optogenetic excitation at SVN evoked a relatively more specific downstream signaling, which primarily recruits the vestibulo-midbrain-cortical and vestibulo-cerebullar pathways. Meanwhile, excitation at MVN evoked a rather diverse downstream signaling via the vestibulo-midbrain-thalamo-cortical, vestibulo-midbrain-thalamo-hippocampal formation, vestibulo-cerebellar, and vestibulo-oculomotor reflex pathways. Altogether, our fMRI findings directly reveal the distinct central vestibular pathways recruited by two different vestibular subnuclei, indicating that organization of vestibular functions arises at the brainstem level.
Discussion and Conclusion
In the present study, we characterized brain-wide functional organizations and downstream activation targets of two major vestibular subnuclei, SVN (Figure 2) and MVN (Figure 3) by optogenetic fMRI. For both SVN and MVN, brain-wide activities were detected by optogenetic excitation at 20Hz. This frequency matched the previously reported range of neuronal firing rates (20-40Hz) of the vestibular nucleus12.Here, our results confirm the indispensable role of the vestibular system in integrating various sensory inputs1-3. We found specific downstream signaling from SVN to auditory-related midbrain and sensorimotor cortices, whereas MVN showed diverse downstream signaling that involves higher order cortices and hippocampal formation. These results indicated that the vestibular system encompasses a more complex organization of its pathways with overlapping inputs from sensory organs and other sensory thalamic nuclei to individual vestibular subnuclei1,13. Furthermore, the unilateral responses evoked by SVN excitatory in the contralateral inferior colliculus and ipsilateral lateral lemniscus demonstrated the unique hemispheric dominance of vestibular activity propagation and the participation of auditory brainstem regions in processing vestibular inputs, which to date has yet to be reported.
Together, our optogenetic fMRI findings directly revealed the distinct brain-wide functional organizations between vestibular subnuclei. These results provide additional insights into where vestibular activities are functionally and dynamically organized brain-wide at the vestibular brainstem level. This optogenetic fMRI approach, in conjunction with future electrophysiological measurements, offers an exciting avenue to further interrogate the vestibular system and its functions.
Acknowledgements
This study was supported by the Hong Kong Research Grant Council (HKU17103819, HKU17104020, R7003-19, C7048-16G, and HKU17112120), Guangdong Key Technologies for Treatment of Brain Disorders (2018B030332001), and Guangdong Key Technologies for Alzheimer’s Disease Diagnosis and Treatment (2018B030336001).References
1. Vidal, P. P. et al. in The Rat Nervous System Ch. 28, 805-864 (Academic Press, 2015).
2. Goldberg, J. M. in Neuroscience in the 21st Century (ed Donald W. Pfaff) Ch. Chapter 30, 883-929 (Springer New York, 2013).
3. Hitier, M., Besnard, S. & Smith, P. F. Vestibular pathways involved in cognition. Front Integr Neurosci 8, 59, doi:10.3389/fnint.2014.00059 (2014).
4. Cullen, K. E. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci 35, 185-196, doi:10.1016/j.tins.2011.12.001 (2012).
5. Shiroyama, T., Kayahara, T., Yasui, Y., Nomura, J. & Nakano, K. Projections of the vestibular nuclei to the thalamus in the rat: APhaseolus vulgaris leucoagglutinin study. The Journal of Comparative Neurology 407, 318-332, doi:10.1002/(sici)1096-9861(19990510)407:3<318::Aid-cne2>3.0.Co;2-h (1999).
6. Lai, H. et al. Morphological evidence for a vestibulo-thalamo-striatal pathway via the parafascicular nucleus in the rat. Brain Res 872, 208-214 (2000).
7. Teune, T. M., van der Burg, J., van der Moer, J., Voogd, J. & Ruigrok, T. J. Topography of cerebellar nuclear projections to the brain stem in the rat. Prog Brain Res 124, 141-172, doi:10.1016/S0079-6123(00)24014-4 (2000).
8. Meng, H., May, P. J., Dickman, J. D. & Angelaki, D. E. Vestibular signals in primate thalamus: properties and origins. J Neurosci 27, 13590-13602, doi:10.1523/JNEUROSCI.3931-07.2007 (2007).
9. Cullen, K. E. & Taube, J. S. Our sense of direction: progress, controversies and challenges. Nat Neurosci 20, 1465-1473, doi:10.1038/nn.4658 (2017).
10. Lee, J. H. et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788-792, doi:10.1038/nature09108 (2010).
11. Leong, A. T. et al. Long-range projections coordinate distributed brain-wide neural activity with a specific spatiotemporal profile. Proc Natl Acad Sci U S A 113, E8306-E8315, doi:10.1073/pnas.1616361113 (2016).
12. Beraneck, M. & Cullen, K. E. Activity of vestibular nuclei neurons during vestibular and optokinetic stimulation in the alert mouse. J Neurophysiol 98, 1549-1565, doi:10.1152/jn.00590.2007 (2007).
13. Lim, R. & Brichta, A. M. in The Mouse Nervous System (eds George Paxinos & Luis Puelles) Ch. 27, 661-681 (Academic Press, 2012).
Figures