2931
Correlation of negative and positive BOLD signal change in the cerebral cortex by somatosensory stimulation in mice1Human Informatics and Interaction Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan, 2NeuroSpin/CEA-Saclay, Gif-sur-Yvette, France
Synopsis
In the current study, we aim to investigate the spatiotemporal profile of negative BOLD response evoked by somatosensory stimulation in mouse. Positive BOLD response was observed in contralateral primary somatosensory cortex (S1) by forepaw somatosensory stimulation, while negative BOLD response was observed in the bilateral barrel field of somatosensory cortex (Scbf). The amplitude of BOLD response in Scbf was negatively correlated with positive BOLD response in S1, while negative BOLD response lasted longer than positive BOLD response. These results indicate that origin of negative BOLD response could be neuronal activity, but neurovascular coupling is not similar as positive BOLD response.
Introduction
Functional MRI (fMRI) is widely used to investigate the neuronal activation. The positive BOLD response reflects the neuronal activation in accordance with the neurovascular coupling. Although negative response has been investigated in animal model so far, there is a controversy about the signal source of the negative BOLD response1,2. In the current study, we aim to investigate spatiotemporal profile of negative BOLD response evoked by somatosensory stimulation in mice.Methods
AnimalsMale C57BL/6 mice (20-30 g) (n = 7) were used.
FMRI experiment
FMRI acquisition was conducted at Bruker 11.7 T with a cryoprobe. fMRI images were acquired using a gradient-echo EPI sequence, TR/TE = 1,000/15 ms, spatial resolution = 100 x 100 x 500 µm3, 15 slices, for 7 min 15 s (435 volumes). Anatomical images were acquired for spatial correction using multi-slice rapid acquisition with relaxation enhancement (RARE) with following parameters, resolution = 100 x 100 x 500 µm3/voxel, TR/effective TE = 2,500/48 ms, RARE factor = 8. Mice were anesthetized with medetomidine (0.05 mg/kg/h, i.p.) and with 0.5% isoflurane following the bolus injection of 0.1 mg/kg medetomidine subcutaneously. The respiration was monitored and the body temperature was maintained at 37 °C during the measurement. Two electrodes (26 gauge) were inserted under the skin of digits 2 and 4 of right forepaw. Electrical pulse stimulation was given with a constant current bipolar isolated pulse stimulator (model 2100; A-M Systems), triggered by a transistor-transistor logic pulse from the Bruker imaging system. The rectangle pulses with 0.3 ms duration, 2 mA current, and 10 Hz were applied five times for 15 s, separated by 60s-rest interval.
Image processing
The slice timing correction, spatial realignment and normalization of functional images were processed by SPM12 (Welcome Trust Center for Neuroimaging, UK). A significant BOLD change map was calculated using SPM12 with a significant level of p < 0.05 (FDR-corrected). Time-course in each brain region was extracted using region of interest (ROI), which was created with reference to Allen mouse brain atlas.
Results
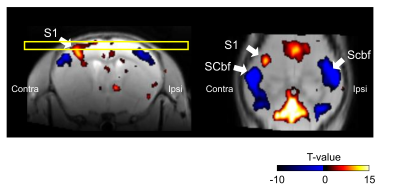
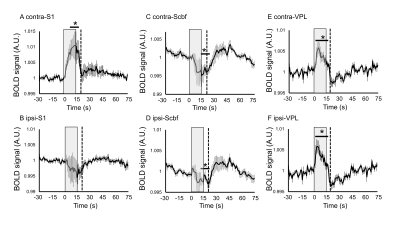
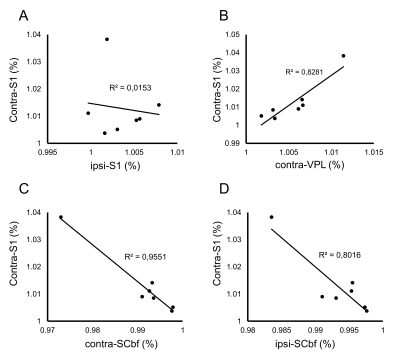
The spatial distribution of positive/negative BOLD response to the somatosensory stimulation was calculated by group analysis. Positive BOLD response was observed in contralateral primary somatosensory cortex (S1) by forepaw somatosensory stimulation, while negative BOLD response was observed in the bilateral barrel field of somatosensory cortex (Scbf) (Fig. 1). The averaged time-courses in the S1, Scbf, and the ventral posterolateral nucleus (VPL) were computed using ROIs (Fig. 2). The averaged BOLD signals in contralateral S1 significantly increased during the somatosensory stimulation and prolonged until 5s after the end of the stimulation (Fig. 2A). The averaged BOLD signals in ipsilateral S1 slightly decreased during the stimulation but it was not significant (Fig. 2B). The averaged BOLD signal changes in bilateral Scbf significantly decreased during the somatosensory stimulation and prolonged until 10s after the end of stimulation (Fig. 2C and 2D). The BOLD signals in bilateral VPL significantly increased during the somatosensory stimulation and prolonged until 5s after the end of the stimulation (Fig. 2E and 2F).The correlation of the amplitudes of BOLD response between distinct brain regions was calculated (Fig. 3). There was no correlation in BOLD responses between contralateral and ipsilateral S1 (Fig. 3A). Importantly, the positive correlation of amplitudes in contralateral S1 and contralateral VPL, which anatomically connects to the S1, was observed (Fig. 3B). The BOLD responses in bilateral barrel field negatively correlated with that in contralateral S1 (Fig. 3C and 3D).
Discussion
In the present study, we investigated spatiotemporal profile of positive and negative BOLD response with somatosensory stimulation in mice. The negative BOLD changes were evoked in bilateral barrel cortices by the somatosensory stimulation and BOLD signals in other regions in the cortex was not changed. Further, the amplitude of BOLD responses among the contralateral S1 and bilateral Scbf was negatively correlated. These results indicate that negative BOLD change in the Scbf could possibly reflect altered neuronal activity, but neurovascular coupling of negative BOLD change is not similar to positive BOLD response.Acknowledgements
No acknowledgement found.References
1. Shmuel A, Augath M, Oeltermann A, Logothetis NK, Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006 Apr;9(4):569-77.
2. Hayes DJ, Huxtable AG, Interpreting deactivations in neuroimaging. Front Psychol. 2012 Feb 7;3:27.
Figures