2929
Functional MRI study of olfactory responses evoked by musk odor in the mouse whole brain under medetomidine anesthesia1Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan
Synopsis
Muscone, a musk odorant component that attracts male mice, binds to olfactory receptors on the olfactory epithelium. Subsequently, activation signals are transferred from the receptors to the olfactory bulb, and then to the olfactory cortex and other brain regions. To detect such odor-evoked activation pathways associated with the perception of odorants and induced behaviors, we employed a method that combines periodic odor stimulation and independent component analysis. In this study, we used medetomidine as the anesthesic. Since medetomidine reportedly influences neural activity in a dose-dependent manner, we investigated the muscone-evoked activations at different levels of medetomidine anesthesia.
Introduction
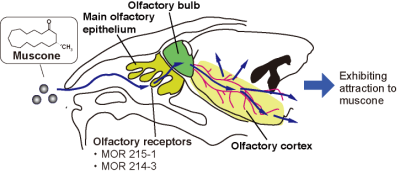
Olfaction is an essential sense for animals and involves brain activation.1 Animals utilize olfaction in avoiding danger, finding food and breeding. We have been studying olfaction in mice by functional MRI. Mice are a useful model in studying animal olfaction, because they have a well-developed olfactory system. Muscone is a major component of musk that attracts male mice,2 and binds to olfactory receptors, MOR215-1 and MOR214-3, on the olfactory epithelium.3 The activation signals are transferred from the receptors to the olfactory bulb, which is the primary center of the olfactory system. The olfactory bulb then relays the signals to the olfactory cortex, as revealed by an immunohistochemistry assay4 (Fig. 1). In order to investigate the olfactory activation pathways in the mouse brain, the BOLD fMRI method is a promising approach. Indeed, the odor-evoked responses in the olfactory bulb have previously been studied by this method.5 However, the mouse brain is small, and thus the detection sensitivity in the BOLD method is low.6 To address this problem, we used a method that combines periodic odor stimulation and independent component analysis (ICA).7 In the method, stimulations are applied at constant intervals. As a result, and task-relevant neural activations occur periodically and are detected by the ICA method. To apply the odor stimulation in a strictly periodic manner, an automated odor stimulation system was constructed.8 In this study, we employed medetomidine as the anesthetic for these experiments. Medetomidine is widely used for functional MRI studies of animals. A previous study of the resting-state functional connectivity of mice demonstrated that medetomidine affected the neural activity in a dose-dependent manner.9 We examined the influence of medetomidine anesthesia on the muscone-evoked activation.Method
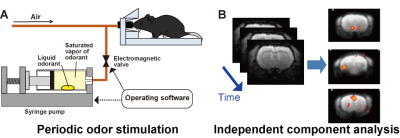
We employed an automated odor stimulation system (in collaboration with the ARCO SYSTEM) to apply periodic odor stimulations to mice.8 In this system, a drop of muscone solution was placed in a syringe pump, and the syringe was filled with the muscone vapor. The syringe pump was then automatically operated to infuse the saturated muscone vapor into the mouse nose (Fig. 2A). MRI experiments were performed with a 7.0 Tesla Bruker BioSpec 70/20 scanner and a mouse brain 2-channel phased array surface cryogenic coil (Bruker BioSpin). Mice (male C57BL/6, 8–10 weeks old) were anesthetized with 1.3% isoflurane and then secured in the MRI cradle. A bolus of medetomidine (0.3 mg/kg) was injected intraperitoneally. Subsequently, different levels of medetomidine were continuously infused, at 0.01, 0.05 and 0.10 mg/kg/h. At 30 min after the bolus injection, the stimulation with muscone was initiated. At 1 min intervals, muscone vapor was applied for 5 sec, and this task was repeated 24 times. During the odor stimulation experiment, GRE-EPI images were acquired: TR/TE = 2000/21.4 ms; FOV = 1.92×1.44 cm2; matrix = 96×72; resolution = 200×200 µm2; slice thickness = 400 µm; number of slices = 19; NEX = 1; flip angle = 70°. Data from three mice were combined and analyzed by ICA (Fig. 2B). The scanned functional data were registered to a template10 and subjected to the group ICA method, using the FSL (FMRIB Software Library; www.fmrib.ox.ac.uk/fsl) program. Signal transition components with the 16.7 mHz frequency were selected as the stimulation-relevant activations, and then the positive components were manually selected. Then, the locations of the components were identified using the annotation map.11Results
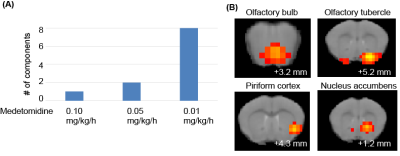
We initially examined the effect of the medetomidine concentration on the muscone-evoked activations. As a result, the number of components detected at the olfactory bulb and the olfactory cortex increased with decreasing concentrations of medetomidine (Fig. 3A). We then focused on the activations detected at the lowest concentration (i.e., 0.01 mg/kg/h), at which a total of 18 activation components were detected in the whole brain. The activated regions included the ventral side of the olfactory bulb, olfactory tubercle, piriform cortex and nucleus accumbens (Fig. 3B).Discussion
Medetomidine is widely used for functional MRI studies. However, medetomidine anesthesia influences the neural activities evoked by muscone (Fig. 3A). This result was qualitatively consistent with the results obtained in a resting-state functional MRI study. The olfactory bulb is the primary center in the olfactory system and relays the activation signals to higher-order brain regions. The muscone-evoked activation on its ventral side was consistent with the immunohistochemical analysis.4 The piriform cortex and olfactory tubercle belong to the olfactory conduction pathway. The muscone-evoked activations detected by immunohistochemistry4 are consistent with those observed in this study. In addition, other activated regions were detected in the whole brain. These regions are potentially associated with behaviors evoked by odor stimulation.Conclusion
We detected muscone-evoked activations in the brains of male mice under medetomidine anesthesia, by the BOLD-ICA method. The muscone-activated regions, detected at low medetomidine concentrations, included the olfactory bulb, olfactory cortex, and other regions.Acknowledgements
The authors gratefully acknowledge Dr. Mika Shirasu and Prof. Kazushige Touhara (The University of Tokyo) for fruitful discussion.References
1. Touhara K, Vosshall LB, Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009;71:307–332.
2. Horio N, et al., Contribution of individual olfactory receptors to odor-induced attractive or aversive behavior in mice. Nat Commun. 1019;10:209.
3. Sato-Akuhara N, et al., Ligand specificity and evolution of mammalian musk odor receptors: Effect of single receptor deletion on odor Detection. J Neurosci. 2016;36:4482-4491.
4. Shirasu M, et al., Olfactory receptor and neural pathway responsible for highly selective sensing of musk odors. Neuron. 2014;81:165-178.
5. Xu F, et al., Odor maps of aldehydes and esters revealed by functional MRI in the glomerular layer of the mouse olfactory bulb. Proc Natl Acad Sci USA. 2003;100:11029–11034.
6, Ielacqua GD, et al., How specific is specific? Stimulus-evoked fMRI in rats and mice. Proc Intl Soc Mag Reson Med. 2015;23:2037.
7. Funatsu H, et al., A BOLD analysis of the olfactory perception system in the mouse whole brain, using independent component analysis. Proc Intl Soc Mag Reson Med. 2017;25:5363.
8. Hayashi F, et al., Odor stimulation by automated syringe pumps in combination with independent component analysis for BOLD-fMRI study of mouse whole brain Proc Intl Soc Mag Reson Med. 2019;27:3685.
9. Nasrallah FA, et al., Neural correlate of resting-state functional connectivity under α2 adrenergic receptor agonist, medetomidine Neuroimage. 2014;84:27-34.
10. Hikishima K, et al., In vivo microscopic voxel-based morphometry with a brain template to characterize strain-specific structures in the mouse brain. Sci Rep. 2017;7:85.
11. Takata N, et al., Flexible annotation atlas of the mouse brain: combining and dividing brain structures of the Allen Brain Atlas while maintaining anatomical hierarchy. bioRxiv org/10.1101/2020.02.17.953547.
Figures