2928
HIGH RESOLUTION ODOR MAPPING IN AWAKE MOUSE OLFACTORY BULB USING CONTRAST ENHANCED FMRI
Christopher Cover1, Alexander Poplawsky1, Sujatha Nallama1, and Mitsuhiro Fukuda1
1Department of Radiology, University of Pittsburgh, Pittsburgh, PA, United States
1Department of Radiology, University of Pittsburgh, Pittsburgh, PA, United States
Synopsis
The rodent olfactory bulb provides an ideal system to investigate cell- and layer-specific contributions to neurovascular coupling using noninvasive fMRI. However, anesthesia is regularly used to minimize motion, which is known to interfere with neurovascular coupling and confounds the neural interpretation of the results. Here, we introduce a technique for reliable awake rodent fMRI of the olfactory bulb at high spatial resolutions (100 x 100 x 300 μm3). Activation maps of four unique odors are consistent with previously published 2-deoxyglucose autoradiography studies. Awake rodent olfactory fMRI is a reliable technique to reproduce neural-specific odor maps at laminar resolutions.
Introduction
The laminar organization of the rodent olfactory bulb and our ability to target specific cells and synapses makes it an ideal system to study the neural basis of functional magnetic resonance imaging (fMRI).1 Typically, anesthesia is used with fMRI to reduce animal motion; but it interferes with neurovascular coupling and confounds the neural interpretation of the results. In the current study, we introduce a technique for high spatial resolution (100 x 100 x 300 μm3) cerebral blood volume weighted fMRI (CBVw-fMRI) of the awake mouse olfactory bulb. To validate this method, we aimed to accurately reproduce known odor-specific activation patterns within the rodent olfactory bulb without the use of anesthesia during scans.Methods
Male B6129SF1/J mice (n = 7, 40.0 ± 2.0 g, mean ± SEM) were used in this study. Mice were implanted with an acrylic head plate and, after sufficient recovery time, conditioned to being head and body restrained within a mock MRI scanner over 3-4 weeks. Prior to imaging, mice were briefly anesthetized with 1.5% isoflurane and injected with monocrystalline iron oxide nanoparticles (MION; 25 mg/kg Fereheme IV) for CBVw-fMRI. Mice were imaged 60 minutes after isoflurane exposure. Imaging was performed on a 9.4 T Bruker scanner with the following EPI acquisition parameters: two segments, TR = 1 s, TE = 7.5 ms, 9 coronal slices. Four odors with known activation profiles were sequentially exposed: amyl acetate (AA), 2-hydroxyacetophenone (2HA), limonene (Lim), and nonanal (Non), in a block design experiment (2-min baseline, 1-min ON, 2-min OFF). Each mouse was exposed to each odor sequence twice in a single scanning session and 2-3 sessions were repeated on different days (n = 19 total sessions). fMRI data were pre-processed and analyzed in AFNI. Data were motion corrected and motion censored for framewise displacement values greater than 25 µm. A general linear model was used to calculate the statistical maps, while a mixed effects meta-analysis calculated group level statistics. To normalize the number of active voxels, only the top 5% of activated voxels (minimum p<0.0001) within the bulb were used in the analysis.Results
The average motion characteristics of the EPI scans after censoring was 11.26 µm ± 1.2 µm as shown in figure 2, with no differences between odors (p=0.52, one-way ANOVA). On average 5% ± 2.5% of data was censored per scan. As shown in figure 1, we reliably observed discrete activation patterns for all four odors, with minimal overlap between odors. Activation patterns were ventral bulb for Non, ventrolateral for Lim, dorsal for 2-HA, and dorsolateral and ventromedial for AA that had some overlap with dorsal and ventral activating odors. These activation patterns are consistent with previously published 2-deoxyglucose autoradiography.2,3 Qualitatively, activation patterns show laminar organization, with the greatest hemodynamic changes occurring along the outer glomerular layer of the olfactory bulb where the olfactory sensory neurons terminate.Discussion
CBVw-fMRI provides researchers with the ability to investigate the laminar activity of the mouse olfactory bulb. Though this study was a proof of concept limited to functional hyperemic responses associated with odor stimulation, this technique was sensitive enough to reproduce specific activation profiles for each odor at high spatial resolutions. This technique could be extended into analyzing cell-specific contributions to neurovascular coupling by integrating molecular techniques, such as optogenetics, to control for cell-specificity. Future studies can couple transgenic mice with molecular techniques to explore neurovascular decoupling in neurodegenerative diseases with olfactory dysfunction, such as Alzheimer’s disease.Conclusion
Awake mouse CBVw-fMRI is a reliable technique to reproduce neural-specific odor maps at high spatial resolutions. We are currently investigating laminar differences in activation profiles.Acknowledgements
No acknowledgement found.References
- Poplawsky AJ, et al. (2015) Layer-specific fMRI response to excitatory and inhibitory neuronal activities in the olfactory bulb. J Neurosci 35(46):15263-75
- Johnson BA, et al. (2002) Functional mapping of the rat olfactory bulb using diverse odorants reveals modular responses to functional groups and hydrocarbon structural features. J Comp Neurol 449(2):180-194.
- Johnson BA, Xu Z, Ali SS, Leon M (2009) Spatial representations of odorants in olfactory bulbs of rats and mice: Similarities and differences in chemotopic organization. J Comp Neurol 514(6):658-673.
Figures
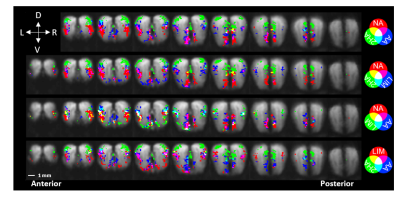
Figure 1. Red-green-blue
color coding of common and discrete CBVw-fMRI activations for each odor with
baseline EPI underlay. Top 5% p-value threshold with a family-wise error
correction of 30 minimum voxels in a cluster. (D – dorsal, V – ventral, R – right,
L – left)
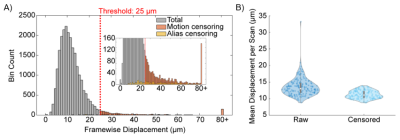
Figure 2. Motion characteristics of all awake fMRI
scans. A) Histogram of all framewise displacement points with values exceeding
a cut-off threshold of 25 μm
(1/4 of a voxel) being highlighted in orange. B) Mean displacement per scan
before and after motion censoring.