2925
Extra low dose pancuronium bromide for fMRI improves survival and recovery times while suppressing translational motion1SBIC, Agency for Science, Technology and Research (A*STAR), Singapore, Singapore
Synopsis
Minimising motion artefacts for pre-clinical studies requires the use of pancuronium bromide (PB), a muscle relaxant paired with the use of mechanical ventilators. Current literature is limited in its description of the mice recovery from the effects of PB. We detail a recovery process using low dose PB which improves survival while keeping translational motion suppressed.
Introduction
Functional magnetic resonance imaging (fMRI) in translational research has become a powerful tool to assess brain connectivity in mouse models1. However, the MR signal is easily contaminated by movement artefacts during data collection. The use of Pancuronium Bromide (PB) as a muscular relaxant paired with the use of mechanical ventilators when conducting fMRI has become common to prevent such artefacts. Established protocols for reliable measurements of functional connectivity have been described with PB bolus and infusion levels ranging from 0.2-0.5 mg/kg and 0.4-0.5 mg/kg/h respectively1-5. However, using the described protocols, we experienced high mortality rates. Given the median lethal dose (LD50) of PB delivered subcutaneously is 0.167 mg/kg6, we hypothesize that a low dose regime below this value would improve survival without compromising motion.Methods
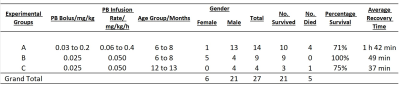
The study was conducted on three separate groups of wild-type mice divided by PB infusion levels, age, and weight: >0.05 mg/kg/h (group A, 6-8 months, N=14), 0.05 mg/kg/h (group B, 6-8 months, N=9). Group C (12-13 months, N=4) used the same doses as group B, but on older obese and control mice (Figure 1).MRI measurements were performed on an 11.7T Bruker BioSpec with CryoProbe. Prior to each fMRI scan, an infusion line was prepared for subcutaneous administration of drugs. The infusion pump was calibrated daily to ensure that volumes dispensed to the infusion line needle had an error of less than 5%.
The mice were induced with 4% isoflurane and underwent endotracheal intubation. The mice were secured to the cradle using ear bars and artificially ventilated at 90 breaths per minute, with isoflurane levels set at 2%; 1:4 O2 to air ratio. Preparation of animals did not exceed 20 minutes. Before moving cradle into the scanner, a medetomidine/PB bolus was administered. Five minutes after bolus injection, isoflurane was reduced to 0.5%. After another five minutes, the mice received a continuous infusion of medetomidine/PB. The medetomidine bolus and infusion levels were maintained at 0.05 mg/kg and 0.1 mg/kg/h respectively for all groups. The bolus and infusion doses of PB for the groups are described in Figure 1. Body temperature was maintained at 36.5±0.5˚C using heated air with feedback from a rectal probe.
Approximately 20mins after bolus injection, BOLD fMRI acquisition with an SSGE-EPI sequence was performed: TE/TR=32/1033ms, FOV=16x8mm2, matrix size=64x32, slice thickness=0.25mm, 35 slices, for a total acquisition time of 10min55s. At the end of the fMRI, infusion was stopped and a structural T2W scan was acquired for 15min 23s.
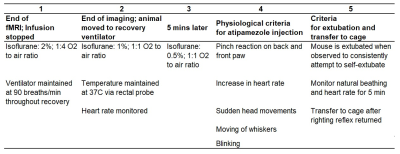
The timeline of the animal recovery process after infusion is stopped is described in Figure 2. Briefly, isoflurane is tapered from 2% to 0.5%; atipamezole is administered when physiological criteria of recovery are met; mouse is extubated when observed trying to self-extubate.
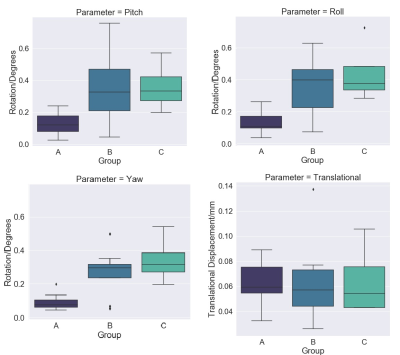
Motion of each subject’s BOLD series was estimated using the preprocessing stage of the Rodent Automated Bold Improvement of EPI Sequences package (RABIES)7. Motion correction was performed with Advanced Normalization Tools8 to yield 6 rigid-body motion parameters (translations dx, dy and dz; rotations pitch, yaw and roll) for every timepoint. Translational parameters were combined into one translational displacement d = (dx2 + dy2 + dz2)½. The maximum value of d, pitch, yaw and roll was recorded for each subject and compared between groups using one-way ANOVA permutation test, with p≤0.05 as significant.
Results
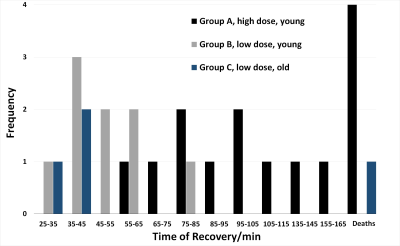
Mice in group A showed a survival rate of 71% and experienced the longest recovery times (mean=102min, range 55-155min). There was also a trend towards decreasing mortality with lower PB dose (data not shown). In group B, survival increased to 100% and we observed a two-fold reduction in total recovery time from end of infusion (mean=49min, range 29-76min) compared with group A. In group C, survival was 75% (one obese mouse died), with mean recovery time of 37min, range 34-39min. A summary of recovery times is shown in Figure 3.One mouse in group A showed an anomalous displacement of >2mm and was excluded as an outlier. For translational motion, there was no significant difference between groups (p=0.9721). The maximum displacement in groups B/C was 0.14mm, which compares well with representative values from a recent multicenter study9. All rotation parameters showed statistically significant differences between higher dose group A vs lower dose groups B/C for pitch (p=0.0031), yaw (p=0.0002) and roll (p=0.0014). However, the maximum rotation in all directions for groups B/C was only 0.76°, with average maximum=0.22°.
Discussion and Conclusion
We have developed an anesthesia and recovery protocol, with reduction in PB dosage by up to 8 times of doses found in literature, that shows similar effectiveness as higher doses in reducing translational motion while reducing recovery times and improving the survival of the mice. Statistically significant difference was found in rotation parameters between higher and lower dose groups. Analysis to determine if this will impact functional maps is ongoing. In group B where mice were young, there were no mortalities. In group C where mice were older, only one obese mouse died. Future studies are needed to determine if even lower doses, or the use of a PB reversal drug such as neostigmine, are needed to improve survival for older, obese mice models.Acknowledgements
Thanks to Sharon Choy, BRC ASTAR for veterinary advice.References
1. Grandjean J, Schroeter A, Batata I, Rudin M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage. 2014;102 Pt 2:838-847. doi:10.1016/j.neuroimage.2014.08.043
2. Grandjean J, Schroeter A, Batata I, Rudin M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage. 2014;102 Pt 2:838-847. doi:10.1016/j.neuroimage.2014.08.043
3. Schroeter A, Grandjean J, Schlegel F, Saab BJ, Rudin M. Contributions of structural connectivity and cerebrovascular parameters to functional magnetic resonance imaging signals in mice at rest and during sensory paw stimulation. Journal of Cerebral Blood Flow & Metabolism. 2017;37(7):2368-2382. doi:10.1177/0271678X16666292
4. Bukhari, Q., Schroeter, A. & Rudin, M. Increasing isoflurane dose reduces homotopic correlation and functional segregation of brain networks in mice as revealed by resting-state fMRI. 2018 Sci Rep 8, 10591. doi:10.1038/s41598-018-28766-3
5. Petrinovic MM, Hankov G, Schroeter A, Bruns A, Rudin M, von Kienlin M, Künnecke B, Mueggler T. A novel anesthesia regime enables neurofunctional studies and imaging genetics across mouse strains. 2016 Sci Rep 6, 24523. doi:10.1038/srep24523
6. National Center for Biotechnology Information. PubChem Compound Summary for CID 27350, Pancuronium bromide. https://pubchem.ncbi.nlm.nih.gov/compound/Pancuronium-bromide. Accessed Dec. 10, 2020
7. RABIES: Rodent Automated Bold Improvement of EPI Sequences. https://github.com/CoBrALab/RABIES
8. Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033-2044. doi:10.1016/j.neuroimage.2010.09.025
9. Grandjean J, Canella C, Anckaerts C, et al. Common functional networks in the mouse brain revealed by multi-centre resting-state fMRI analysis Neuroimage. 2020 Jan 15;205:116278. doi: 10.1016/j.neuroimage.2019.116278