2922
Optimize Simultaneous Multi-channel Calcium Recording and fMRI in Mouse Brain1Queensland Brain Institute, Brisbane, Australia, 2Quuensland Brain Institute, Brisbane, Australia, 3Center for Advanced Imaging, Brisbane, Australia
Synopsis
· Susceptibility artifacts of EPI induced by fiber photometry were successfully reduced at 9.4T MRI.
· High quality event-related BOLD and calcium responses were measured using short visual stimuli.
· Neural to hemodynamic transfer functions of a cortical region (V1) and a subcortical region (LGd) were calculated.
Introduction
Simultaneous calcium recording can provide information on neural activity that underlies functional MRI response 1–4. Genetically encoded calcium indicator, such as GCaMP6f, allows cell-type specificity 5–7; therefore this method can be used to understand the contributions of different cell populations to BOLD signal. However, simultaneous fiber photometry recording in mouse brain is particularly challenging due to strong susceptibility artefact induced by the fiber cannula and dental cement on EPI at ultrahigh field. The artifact is worsen when multiple fibers are implanted to target different areas. In this study, we stablished a method to minimize the susceptibility artefact to enable high-quality calcium recording and ultrafast fMRI from cortical and subcortical areas.Methods
The study was approved by animal ethic committee of the University of Queensland. To minimize EPI artifact 8,9, we evaluated 1) fiber cannula of 200, 100 and 60 micron diameters; 2) two types of dental cements, and 3) two susceptibility-match covering materials over the head. We injected AAV-hSyn-GCaMP6f into visual cortex (V1) and lateral-genicular-nucleus (LGd) of 8-9 weeks old C57BL6/J mice. Two optic fiber cannulas were implanted and secured with dental cement. 4 weeks after surgery, simultaneous calcium and fMRI were done under 0.1mg/kg/h medetomidine and 0.1-0.3% isoflurane for anaesthesia. Evoked responses to visual stimuli (3s duration, 5Hz, 12s intervals, and 15trials) were recorded by photometry (Neurophotometric, USA) and 9.4T MRI with a 10mm single loop receiver coil (Bruker). Calcium recording was done using alternating excitation (470nm) and reference (410nm) lights with frame rate of 20Hz (10Hz for each channel). Ultrafast multiband EPI 10 (TR=0.3s, TE=15ms) was used to acquire BOLD fMRI of whole brain with voxel size of 0.3×0.3×0.6 mm3. fMRI processing was done using FSL, AFNI, and MATLAB. Calcium signal was detrend and regressed by the reference light. BOLD time-courses were obtained by drawing 3×3 voxels ROIs in two slices over the targeted areas.Results
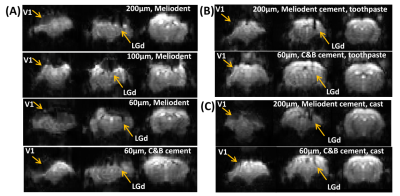
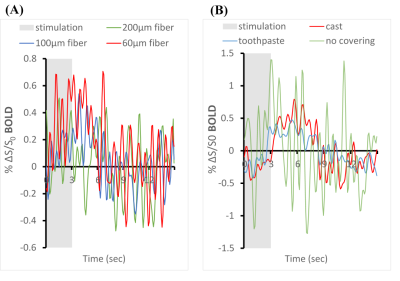
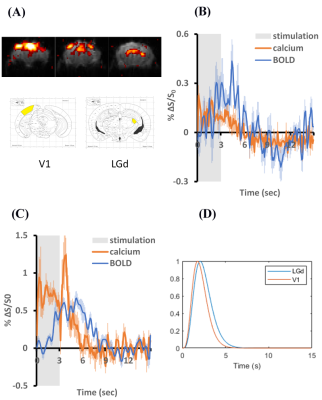
Figure 1 shows the effects of fiber diameters, dental cement types, and covering materials on susceptibility artefact in GE-EPI. The commonly used 200 µm fiber caused a signal loss of up to 3 voxels along the fiber. Thin fiber of 60µm could minimize such signal loss in LGd. By applying toothpaste or kwik-cast as covering material signal loss over the cortex could be eliminated. Figure 2A shows improved BOLD signal in LGd by decreasing fiber diameter. Figure 2B shows good BOLD signal with both covering materials. Figure 3 shows consistent calcium and BOLD responses in V1 and LGd. The transfer functions from calcium to BOLD, estimated using gamma variate functions, show slightly different timing characteristics (Figure 3D).Discussion
EPI image quality was drastically improved by decreasing the magnetic field inhomogeneities around cement-air interface with a covering material. Kwik-cast is more effective than toothpaste in reducing the artefact. It is also more durable for long-term studies. Although using a thinner fiber decreases the signal loss (Figure 1), the fluorescent light that can be collected will decrease as well. Thus, a balance should be found between MRI image quality and calcium signal. We found strong calcium signal cloud be recorded with 60µm fiber. Both types of dental cements induced similar artifacts. We chose C&B because it could fixate the implants for a longer time. Reducing the fiber size can improve the BOLD signal but, the BOLD signal in V1 is still too noisy without a covering material. Therefore, the combination of thin fiber optic and proper covering material would be the best approach. The calcium recording in LGd was less successful compared to V1 due to the smaller size. Interestingly, there was an ‘off-response’ after stimulation in calcium response. This phenomenon was consistent even when calcium-independent (non-neuronal) signal was eliminated by reference, and when stimulation intensity was reduced to 1/10 to avoid any after-effect of strong light. In summary, this study established a method to measure high quality BOLD and calcium activation in mouse with multiple implants. This enables studying functional connectivity in the future.Acknowledgements
No acknowledgement found.References
1. Schulz, K. et al. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat. Methods 9, 597–602 (2012).
2. Schmid, F. et al. Assessing sensory versus optogenetic network activation by combining (o)fMRI with optical Ca2+recordings. J. Cereb. Blood Flow Metab. 36, 1885–1900 (2016).
3. Liang, Z., Ma, Y., Watson, G. D. R. & Zhang, N. Simultaneous GCaMP6-based fiber photometry and fMRI in rats. J. Neurosci. Methods 289, 31–38 (2017).
4. Wang, M., He, Y., Sejnowski, T. J. & Yu, X. Brain-state dependent astrocytic Ca2+ signals are coupled to both positive and negative BOLD-fMRI signals. Proc. Natl. Acad. Sci. U. S. A. 115, E1647–E1656 (2018).
5. Cui, G. et al. Deep brain optical measurements of cell type-specifc. Nat. Protoc. 9, 1213–1228 (2014).
6. Schlegel, F. et al. Fiber-optic implant for simultaneous fluorescence-based calcium recordings and BOLD fMRI in mice. Nat. Protoc. 13, 840–855 (2018).
7. Looger, L. L. & Griesbeck, O. Genetically encoded neural activity indicators. Curr. Opin. Neurobiol. 22, 18–23 (2012).
8. Aue, S. P. Susceptibility artifacts on spin-echo and gradient-echo images. 492, 473–492 (1990).
9. Farahani, K., Sinha, U., Sinha, S., Chiu, L. C. L. & Lufkin, R. B. Effect of field strength on susceptibility artifacts in magnetic resonance imaging. Comput. Med. Imaging Graph. 14, 409–413 (1990).
10. Lee, H. L., Li, Z., Coulson, E. J. & Chuang, K. H. Ultrafast fMRI of the rodent brain using simultaneous multi-slice EPI. Neuroimage 195, 48–58 (2019).
Figures