2919
Accurate Brain Parcellation of Individual Marmosets Based on Awake Resting-State fMRI Data and Deep Neural Networks1Dept. of Neurobiology, University of Pittsburgh, Pittsburgh, PA, United States, 2Institute of Neuroscience, Chinese Academy of Sciences, Shanghai, China
Synopsis
As a prerequisite for understanding how the brain works, it has been a long-sought goal to subdivide (parcellate) the brain into a mosaic of anatomically- and functionally-defined parcels (areas). However, reaching a consensus parcellation has been hindered by inaccuracies in aligning brain areas across subjects. Here, we developed a novel cortical parcellation approach using resting-state fMRI data collected in a population of awake marmosets to accurately map the functional brain organization of individuals.
INTRODUCTION
In the neuroscience field, a prerequisite for understanding the complex organization of the cerebral cortex is to accurately map (or parcellate) its subdivisions, known as cortical areas [1]. Although it has been a century-old objective, brain parcellation has been plagued by many problems, such as the number of cortical subdivisions and whether the same subdivisions can be identified in different individuals. In the present work, we collected resting-state functional magnetic resonance images (rsfMRI) data from a population of 42 healthy common marmosets, a New-World monkey of ever increasing interest in neuroscience that has many advantages as a subject for neuroimaging techniques, including a lissencephalic brain. The group-average rsfMRI data provided an accurate template of the functional organization of the cortex. This map was compared with different maps of the marmoset brain [2] and its accuracy was further validated using anatomical neuronal tracing data [3]. From the population-averaged functional parcellation, we also applied a deep-learning neural network to learn the ‘fingerprint’ of each cortical subdivision and enable the identification of these cortical areas in each individual. Our new parcellation pipeline and classifier provides significant improvements over existing parcellation methods, and can be a useful tool to understand the structural and functional architecture of the primate cerebral cortex and its variability across individuals. The method can be extended to other primate species, including humans.METHODS
Data collection and Preprocessing: We acquired test-retest resting-state fMRI datasets from a population of marmosets in the National Institute of Health (NIH, Bethesda, USA) and the Institute of Neuroscience (ION, Shanghai, China) comprised of 42 marmosets, 62 sessions and 721 runs (17 min per run). A similar animal training and MRI scanning protocols was applied both at the NIH and ION to maximize the compatibility and consistency of the two datasets. The rsfMRI data was acquired with a 0.5-mm isotropic spatial resolution and a temporal resolution (TR) of 2 sec. Data preprocessing involved slice-timing correction, motion correction, EPI-distortion correction, band-pass filtering, and noise-signal regression. The preprocessed data were spatial normalized to the template space of the population-based Marmoset Brain Mapping Atlas V3 [4]. Group-ICA were performed to identify several different brain networks in the marmoset population, using different number of component settings. All components from the Group-ICA were manually classified and the network components were manually converted into a brain-network parcellation map. We used the resting-state functional boundary maps proposed by Gordon et al. [5] to define subdivisions (parcels) that represented putative cortical areas. Then, we assigned a network color to different parcels based on their spatial overlap. The semi-automated neuroanatomical approach described above was used to identify the functional subdivisions (parcels) of the population-averaged brain. We then developed an automated approach for identifying the corresponding parcellation in each individual based on a classifier comprised of a deep-learning neural network. In our case, the classifier learned the ‘areal fingerprint’ of each cortical subdivision (parcel) that distinguished it from its surroundings. Based on the learning of the population multi-modal feature maps which contained areal properties of architecture, function, connectivity, and topography, the areal classifier returned a good prediction of the different cortical subdivisions for each individual subject. Lastly, we performed specific studies to quantify the reliability of each subdivision in our defined map [6], as well as validate the parcellation against anatomical neuronal tracing results of marmosets [3].RESULTS AND CONCLUSION
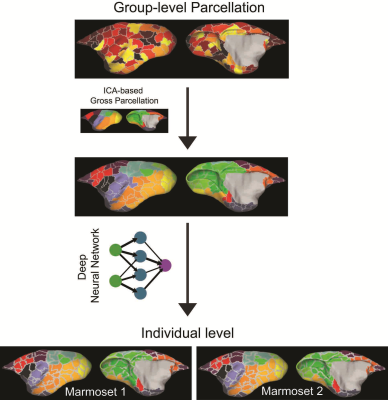
Using the methods of Gordon et al. [5], we identified 132 independent cortical parcels in the marmoset cortex. Figure 1 shows the group-level brain parcellation (first row) and the networks identified by ICA (second row). The third row in Fig. 1 shows cortical parcels assigned to different networks based on their spatial overlapping. Using a deep-learning neural network, we successfully identified the different cortical areas in individual subjects. The bottom row shows the cortical parcellations in 2 different individuals. All individuals retained a high reproducibility with the population. However, clear individual differences in cortical parcellation can be noticed (e.g., compare Marmoset 2 against Marmosets 1).In summary, we developed a novel method to produce a highly-reproducible cortical parcellation of the marmoset cortex based on analysis of population-averaged rsfMRI data. ICA analysis allowed the assignment of the different cortical parcels to brain networks. Training and use of a deep-learning network allowed the identification of the cortical parcels in each individual. Our method assures a stable and reproducible assignment of individual cortical areas in the marmoset brain. The method can be easily generalized to segment the cortex of other primate species, including macaques and humans.
Acknowledgements
This research was supported, in part, by the Intramural Research Program of the NIH, NINDS (ZIA NS003041), by the PA Department of Health SAP #4100083102 to ACS, and by the startup grant of CAS Center for Excellence in Brain Science and Intelligence Technology to CL and ZL.
References
1. Eickhoff SB, Yeo BTT, Genon S. Imaging-based parcellations of the human brain. Nat Rev Neurosci 19, 672-686 (2018).
2. Liu C, et al. A digital 3D atlas of the marmoset brain based on multi-modal MRI. Neuroimage 169, 106-116 (2018).
3. Majka P, et al. Open access resource for cellular-resolution analyses of corticocortical connectivity in the marmoset monkey. Nat Commun 11, 1133 (2020).
4. Liu C, Yen C-C, Szczupak D, Tian X, Glen D, Silva AC. Marmoset Brain Mapping V3: Population multi-modal standard volumetric and surface-based templates. Neuroimage, (2021).
5. Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex 26, 288-303 (2016).
6. King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, Diedrichsen J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci 22, 1371-1378 (2019).