2910
fMRI with a Zero Echo Time (ZTE) Pulse Sequence1Center for Animal MRI, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Biomedical Research Imaging Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 3The Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 4Department of Neurology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 5Department of Radiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Synopsis
Conventional fMRI studies, carried out with the gold-standard echo-planar imaging (EPI), are confounded by the deleterious effects of the sequence’s limitation – its sensitivity to magnetic field inhomogeneities and high acoustic noise. The properties of short acquisition delay sequences, that also have minimal incrementing of gradients during spatial encoding, such as MB-SWIFT and ZTE, render them resistant to the aforementioned confounding factors. We study the feasibility of using ZTE to detect functional activations with endogenous contrast using a simple rat forepaw electrical stimulation paradigm. We show that ZTE-fMRI has a 67% greater sensitivity than the gold-standard BOLD-weighted EPI.
Introduction
Conventional fMRI studies, carried out with the gold-standard echo-planar imaging (EPI), are confounded by the deleterious effects of the sequence’s limitations[1,2] – its sensitivity to magnetic field inhomogeneities and high acoustic noise [3,4] The properties of short acquisition delay sequences, that also have minimal incrementing of gradients during spatial encoding, such as MB-SWIFT[5] and ZTE[6], render them resistant to the aforementioned confounding factors associated with EPI.The pioneers of short acquisition delay fMRI have demonstrated pronounced reduction in sensitivity to magnetic susceptibility and motion induced artifacts and acoustic noise[7,8] with a MB-SWIFT sequence. To date, no short acquisition delay sequence has yet to demonstrated superior sensitivity to gold standard EPI sequences[9]. We previously showed that functional imaging with a ZTE pulse sequence was possible with the aid of intravascular contrast agents [10] however we were unsure if ZTE-fMRI without a contrast agent would be sensitive to functional activations and, if so, what the contrast mechanism would be.
The present study aims to further address these issues, utilizing a ZTE pulse sequence with rat forepaw electrical stimulation. Our hypothesis was that following modeling of ZTE fMRI signal and optimization of reconstruction algorithm that ZTE-fMRI would be able to detect functional activations through an endogenous contrast means at a greater sensitivity than the gold-standard BOLD-weighted fMRI. Successful implementation of ZTE-fMRI will help push the current boundaries of fMRI and, alongside MB-SWIFT, open up a new avenue for distortion insensitive, quiet fMRI with superior sensitivity.
Methods
Data were acquired with a Bruker BioSpec 9.4T/30-cm system (Bruker Corp., Billerica MA) with a BFG-240/120 gradient insert (RRI., Billerica MA) using a homemade transceiver coil.ZTE imaging parameters: TR-0.9 ms, acquisition bandwidth-100 kHz, excitation bandwidth/RF pulse length – 320kHz/4μs , FOV-40mm 3, Matrix size – 60 3, 3334 projections per volume, yielding a 3 s temporal resolution. BOLD-weighted EPI imaging parameters: TR-3,000ms, TE- 14 ms acquisition bandwidth - 300 kHz, FOV - 40mm 2, matrix size – 60 2 , slice thickness – 0.67 mm, slices – 32.
ZTE fMRI reconstruction algorithms were compared using the BART reconstruction toolbox [11]. Online reconstruction, comprising of algebraic reconstruction and gridding in 3D k-space [12], was carried out by ParaVision 6.0.1.
Forepaw stimulations electrical stimulations comprised an off-on off paradigm: 60-30-180 s, repeated 3 times with a constant current at 2 mA, pulse width – 0.5 ms and frequency – 9 Hz (n=3 subjects, 27 trials for ZTE-fMRI and BOLD).
Analysis was carried out with in-house scripts using python. 3 voxel3 ROIs were extracted from contralateral SI for evoked response analysis.
Results and Discussion
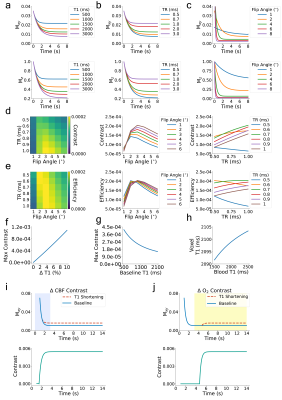
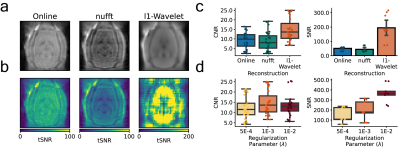
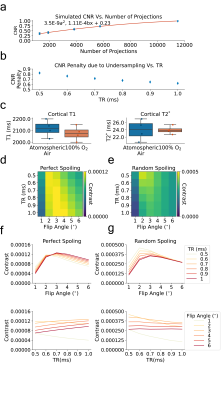
To elucidate a range of ZTE-fMRI imaging parameters we carried out Bloch equation analysis, initially assuming perfect spoiling. Fig1.a-c show how the transverse and longitudinal components of magnetization vary as a function of baseline T1 value, TR and flip angle respectively. Fig1.d shows the modeled functional contrast assuming a 2%ΔT1 from a baseline of 2,100 ms. Fig1.e Is the contrast as a function of TR and flip angle divided by an efficiency term, taken sqrt(TR). Together, Fig.1d and Fig.1e demonstrate that functional contrast with ZTE is achievable within the sequence’s limitations[13]. Fig.1f shows that a linear relationship exists between maximum contrast with flip angle and TR range of 1-6° and TRs of 0.5-1ms respectively. Fig.1g indicates that the achievable functional contrast may be heightened at shorter baseline T1 values. Assuming BVF of 5% and maximum dilation during of vessels during functional activation of 20%, Fig.1h demonstrates that CBV changes do not contribute to ZTE fMRI functional activations as voxel T1 is relatively insensitive to large changes in blood T1. Fig.1i shows how changes in blood flow could contribute to evoked response contrast when spins are excited in a dynamic state. However, we do not expect that in flow contributes to the ZTE fMRI signal. ZTE employs a non-selective RF pulse thus spins from the regional acceleration of blood flow will have reached a pseudo-steady state [6]. We believe that that ZTE-fMRI is sensitive to the increase in tissue oxygenation [14] which shortens the R1 of spins in a pseudo steady-state Fig.1j, as preliminary experiments indicate that oxygen challenge induces similar tissue oxygenation changes as stimulus evoked action.To improve ZTE data, we investigated the use of different reconstruction algorithms. Fig2.a-b shows raw functional ZTE data reconstructed online, with the nufft, and with l1-wavelet regularization and the corresponding tSNR maps. Fig.2c-d compares CNR and SNR of data reconstructed with different algorithms and the effect of l1-wavelet regularization parameter on CNR of evoked response. CNR and SNR of functional data are likely optimal with l1-wavelet regularization, as this process suppresses additive white noise [15].
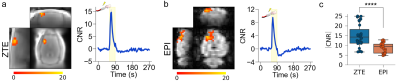
Finally, we compared BOLD-weighted and ZTE-fMRI responses, reconstructed with l1-wavelet a regularization parameter of 1x10-3, and demonstrated a 67% higher CNR than BOLD-weighted EPI Fig.4.
With the technique's previously demonstrated marked reduction in sensitivity to magnetic field inhomogeneities, motion and inaudible acoustic noise[10] along with its superior sensitivity to functional responses; ZTE-fMRI is not only an ideal technique for rodent fMRI but for whole functional neuroimaging field.
Acknowledgements
We thank Mark Mattingly for his advice regarding gradient system operation which aided sequence programming.
We thank UNC CAMRI members for their helpful discussions and critiques.
This work is supported in part by NIH grants RF1MH117053, R01MH111429, R01NS091236, P60AA011605, and U54HD079124.
References
1. Haacke EM (1999) Magnetic resonance imaging : physical principles and sequence design. Wiley, New York
2. Hong X, To XV, Teh I, Soh JR, Chuang KH (2015) Evaluation of EPI distortion correction methods for quantitative MRI of the brain at high magnetic field. Magn Reson Imaging 33 (9):1098-1105. doi:10.1016/j.mri.2015.06.010
3. Bonhomme V, Boveroux P, Brichant JF, Laureys S, Boly M (2012) Neural correlates of consciousness during general anesthesia using functional magnetic resonance imaging (fMRI). Arch Ital Biol 150 (2-3):155-163. doi:10.4449/aib.v150i2.1242
4. Tang CY, Ramani R (2016) fMRI and Anesthesia. Int Anesthesiol Clin 54 (1):129-142. doi:10.1097/AIA.0000000000000081
5. Idiyatullin D, Corum CA, Garwood M (2015) Multi-Band-SWIFT. J Magn Reson 251:19-25. doi:10.1016/j.jmr.2014.11.014
6. Weiger M, Brunner DO, Dietrich BE, Muller CF, Pruessmann KP (2013) ZTE imaging in humans. Magn Reson Med 70 (2):328-332. doi:10.1002/mrm.24816
7. Lehto LJ, Idiyatullin D, Zhang J, Utecht L, Adriany G, Garwood M, Grohn O, Michaeli S, Mangia S (2017) MB-SWIFT functional MRI during deep brain stimulation in rats. Neuroimage 159:443-448. doi:10.1016/j.neuroimage.2017.08.012
8. Paasonen J, Laakso H, Pirttimaki T, Stenroos P, Salo RA, Zhurakovskaya E, Lehto LJ, Tanila H, Garwood M, Michaeli S, Idiyatullin D, Mangia S, Grohn O (2020) Multi-band SWIFT enables quiet and artefact-free EEG-fMRI and awake fMRI studies in rat. Neuroimage 206:116338. doi:10.1016/j.neuroimage.2019.116338
9. Kim MJ, Jahng GH, Lee SY, Ryu CW (2013) Functional magnetic resonance imaging with an ultrashort echo time. Med Phys 40 (2):022301. doi:10.1118/1.4773035
10. MacKinnon MJ, Song, S., Hsu, L., Lee, S., Johnson, A., Shih, Y. (2019) i-ZTE fMRI. Paper presented at the Proc. Intl. Mag. Reas. Med.,
11. Uecker M, Ong, F., Tamir, J, I., Bahri, D., Virtue, P., Cheng, J. Y., Zhang, Tao., Lustig, M. Berkeley Advanced Reconstruction Toolbox. In: Proc. Intl. Soc. Mag. Reson. MEd. , 2015. p 2802
12. Weiger M, Hennel F, Pruessmann KP (2010) Sweep MRI with algebraic reconstruction. Magn Reson Med 64 (6):1685-1695. doi:10.1002/mrm.22516
13. Schieban K, Weiger M, Hennel F, Boss A, Pruessmann KP (2015) ZTE imaging with enhanced flip angle using modulated excitation. Magn Reson Med 74 (3):684-693. doi:10.1002/mrm.25464
14. Zhou H, Chiguru S, Hallac RR, Yang D, Hao G, Peschke P, Mason RP (2019) Examining correlations of oxygen sensitive MRI (BOLD/TOLD) with [(18)F]FMISO PET in rat prostate tumors. Am J Nucl Med Mol Imaging 9 (2):156-167
15. Choi HH, Lee JH, Kim SM, Park SY (2015) Speckle noise reduction in ultrasound images using a discrete wavelet transform-based image fusion technique. Biomed Mater Eng 26 Suppl 1:S1587-1597. doi:10.3233/BME-151458
Figures