2905
Visualizing Human Aortic Valve Opening and Closing with Sub-Millisecond Temporal Resolution Over a Reduced Field-of-View1CMRR, University of Illinois at Chicago, Chicago, IL, United States, 2Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 3Neurosurgery, University of Illinois at Chicago, Chicago, IL, United States, 4Radiology, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
An imaging technique, coined epi-based Sub-millisecond Periodic Event Encoded Dynamic Imaging, or epi-SPEEDI, has been shown capable of visualizing the rapid opening and closing of human aortic valve with sub-millisecond temporal resolution. However, the acquisition time of epi-SPEEDI was relatively long (8-10 breath holds). Herein, we report an alternative SPEEDI technique with a reduced FOV, which we call rFOV-SPEEDI, to shorten the scan times. This technique has been successfully applied to visualization of human aortic valve opening and closing with a temporal resolution of 0.6 ms, together with a scan time reduction of 32.5%.
Introduction
Visualization of the rapid opening and closing of the human aortic valve is important in detecting valvular diseases such as stenosis and regurgitation. This has been clinically done using ultrasound echography due to the limited temporal resolution of MRI and the rapid movement of valvular structures. Recently, a novel MRI technique, coined epi-based Sub-millisecond Periodic Event Encoded Dynamic Imaging, or epi-SPEEDI1, was proposed and successfully used to capture the dynamic process of aortic valve opening and closing with a temporal resolution of 0.6ms. However, the acquisition time of epi-SPEEDI is relatively long (8-10 breath holds), which can potentially introduce position inconsistency among different breath holds. One approach to shortening the acquisition time is to limit the field-of-view (rFOV), thereby reducing the number of phase-encoding steps while maintaining the spatial resolution, as demonstrated in other applications2–4. This approach is particularly suitable for aortic valve imaging where only a small FOV is needed. Herein, we report an rFOV SPEEDI technique, which we call rFOV-SPEEDI, to shorten the scan times and demonstrate its ability to visualize the human aortic valve opening and closing with a sub-millisecond temporal resolution.Methods
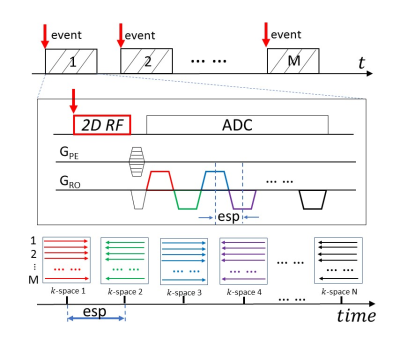
rFOV-SPEEDI SequencerFOV-SPEEDI is built upon an epi-SPEEDI pulse sequence reported recently1. By synchronizing with a cyclic event under investigation (Figure 1), N gradient echoes from the echo-train in epi-SPEEDI are employed to sample N separate k-space matrices, each corresponding to a distinct time point. The echo-train acquisition is repeated M times, producing N resultant k-space matrices that are individually reconstructed to generate a series of time-resolved images. The temporal resolution is determined by the echo spacing (esp), which is typically shorter than 1ms. By replacing the conventional excitation RF pulse in epi-SPEEDI with a custom 2D RF pulse, rFOV-SPEEDI was able to reduce the scan times by limiting the FOV and decreasing the number of required phase-encoding steps (or M) accordingly.
Data Acquisition and Image Analysis
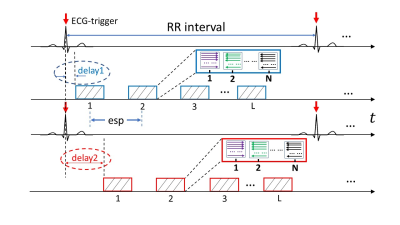
With IRB approval, aortic valve images were acquired from healthy subjects on a 3T GE MR750 scanner using both rFOV-SPEEDI and full FOV epi-SPEEDI with EKG signals as a trigger. To capture the entire opening and closing processes of the aortic valve, an “interleaved multi-phase” acquisition scheme was used with multiple trigger delays as shown in Figure 2. The temporal gaps in-between the acquisition blocks were filled up using L acquisition blocks acquired from different trigger delays (e.g., the blue acquisition blocks can be dovetailed by the red acquisition blocks). Three trigger delays (18ms/28ms/38ms) were used in the rFOV-SPEEDI scan with a total acquisition time of 108 heartbeats in 5-6 breath-holds. The other key sequence parameters for rFOV-SPEEDI acquisition were: TR/TE=29/8.8ms, flip angle = 10º, slice thickness=8mm, FOV=12cm×12cm, matrix size=60×60, esp=0.6ms, L=20 per R-R interval, N=16 per excitation, and M=36. For full FOV acquisition, the imaging parameters were identical to those used in the rFOV-SPEEDI sequence, except for TR/TE=20/3ms, FOV=24cm×24cm, matrix size=118×118, L=32, M=80, trigger delays=12ms/22ms, and acquisition time = 160 heartbeats in 8-10 breath-holds.
The acquired k-space data from both acquisitions was reconstructed offline using customized Matlab programs. The reconstructed images were reordered according to their acquisition time based on the acquisition strategies described in Figure 2. To monitor the dynamic change of the aortic valve, the planimetric aortic valve area (AVA) was extracted from each image in the time series.
Results
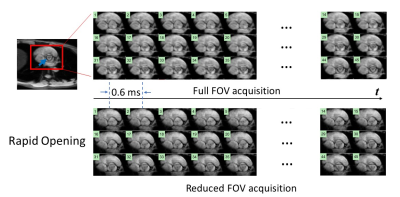
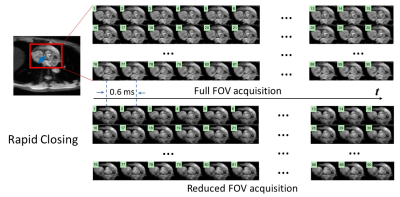
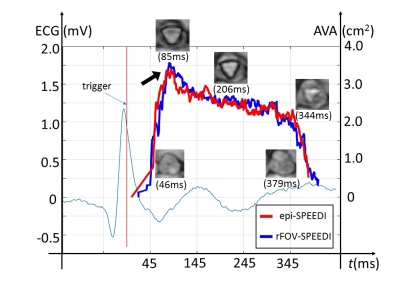
Figures 3 and 4 show two sets of time-resolved images, both with a temporal resolution of 0.6 ms, during the rapid opening and rapid closing phases of the aortic valve from a representative healthy human subject (male; 30-years-old). The dynamics were clearly visualized from both full FOV and reduced FOV acquisitions. Compared with the full FOV acquisition, rFOV-SPEEDI reduced the scan time by 32.5% (108 vs. 160 heartbeats) without compromising the image quality.Figure 5 displays two planimetric AVA dynamic curves obtained from the full FOV acquisition and reduced FOV acquisition, both with the temporal resolution of 0.6ms, together with the ECG waveform from the subject. The two AVA curves matched well with each other. From either AVA curve, the three phases during the aortic valve opening and closing can be well identified: a rapid opening phase, a slow closing phase, and a rapid closing phase. In addition, both AVA curves revealed an overshoot when the aortic valve opened maximally, which was reported in the literature using non-MRI techniques5,6.
Discussion and Conclusion
Using rFOV-SPEEDI, we have experimentally observed the dynamics of aortic valve rapid opening and closing in human subjects with sub-millisecond temporal resolutions and adequate spatial resolutions in a reduced scan time of 108 heartbeats. This scan time can be shortened further by combining rFOV-SPEEDI with parallel imaging such as SENSE7, GRAPPA8, low-rank9, sparse matrix decomposition10, machine learning11, and other sparse k-space sampling methods. Based on the demonstration herein, the rFOV-SPEEDI technique may find other applications for visualizing ultrafast and cyclic biological and physical processes, especially in situations where the region of interest is in a focal area within a larger object.Acknowledgements
This work was supported in part by a grant from the National Institutes of Health (Grant No. NIH 1S10RR028898). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors are grateful to Dr. Yi Sui of Mayo Clinic for initially designing the 2D RF pulse.References
1. Zhong, Z., Sun, K., Dan, G., Karaman, M. M. and Zhou, X. J. Capture the Opening and Closing of Human Aortic Valve Using MRI with Sub-Millisecond Temporal Resolution. in Proc. Intl. Soc. Magn. Reson. Med. (2020).
2. Zhong, Z. et al. High-Spatial-Resolution Diffusion MRI in Parkinson Disease: Lateral Asymmetry of the Substantia Nigra. Radiology 291, 149–157 (2019).
3. Finsterbusch, J. Fast-spin-echo imaging of inner fields-of-view with 2D-selective RF excitations. J. Magn. Reson. Imaging 31, 1530–1537 (2010).
4. Zhou, X. J. and Sui, Y. Image Domain Segmented Echo Planar Magnetic Resonance Imaging Using a 2D Excitation Radiofrequency Pulse. US Patent No. 9797970 (2016).
5. Laniado, S., Yellin, E., Terdiman, R., Meytes, I. and Stadler, J. Hemodynamic correlates of the normal aortic valve echogram. A study of sound, flow, and motion. Circulation 54, 729–737 (1976).
6. van Steenhoven, A. A., Veenstra, P. C. and Reneman, R. S. The effect of some hemodynamic factors on the behaviour of the aortic valve. J. Biomech. 15, 941–950 (1982).
7. Pruessmann, K. P., Weiger, M., Scheidegger, M. B. and Boesiger, P. SENSE: sensitivity encoding for fast MRI. Magn. Reson. Med. 42, 952–962 (1999).
8. Griswold, M. A. et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn. Reson. Med. 47, 1202–1210 (2002).
9. Lingala, S. G., Hu, Y., DiBella, E. and Jacob, M. Accelerated Dynamic MRI Exploiting Sparsity and Low-Rank Structure: k-t SLR. IEEE Trans. Med. Imaging 30, 1042–1054 (2011).
10. Otazo, R., Candès, E. and Sodickson, D. K. Low-rank plus sparse matrix decomposition for accelerated dynamic MRI with separation of background and dynamic components: L+S Reconstruction. Magn. Reson. Med. 73, 1125–1136 (2015).
11. Han, Y., Sunwoo, L. and Ye, J. C. k-Space Deep Learning for Accelerated MRI. ArXiv180503779 Cs Stat (2019).
Figures