2904
Cardiac Self-Gating for Free-Breathing Phase-Contrast MRI1Department of Pediatrics, Harvard Medical School, Boston, MA, United States, 2Department of Cardiology, Boston Children's Hospital, Boston, MA, United States, 3Department of Informatics, Technical University of Munich, Munich, Germany, 4Munich School for Data Science, Munich, Germany, 5Philips Healthcare, Gainesville, FL, United States
Synopsis
We implemented an end-to-end cardiac self-gating algorithm for a novel free-breathing 2D cine phase-contrast sequence on a clinical MRI scanner. We validated our technique against clinically performed ECG-gated cine phase-contrast scan. Strong agreement with no statistically significant difference was shown between the systemic and pulmonary blood flow measurements calculated between the standard ECG-gating and self-gating scans. Our approach eliminates the need for an ECG signal and allows for blood flow measurements where an ECG is not accessible such as fetal cardiac MRI.
Introduction
Phase-contrast MRI provides accurate measurements of the cardiovascular blood flow and is a useful tool to assess shunts and valvular diseases. In addition, it may help diagnose congenital heart disease and guide the timing of an intervention in the study of fetal hemodynamics.1 However, the clinical standards for adults cannot be translated to fetuses without modifications. Clinically, cardiac scans are gated using an electrocardiogram (ECG) to resolve for cardiac motion. Given that the data is acquired over multiple cardiac cycles, each chunk of data needs to be binned into its respective heart phase, such that images for a discrete number of cardiac phases can be constructed. ECG cannot be recorded from the fetal heart and suffers from increasing artifacts in higher magnetic field strengths.2 To address these shortcomings, cardiac self-gating (SG) attempts to track cardiac motion by retrospectively analyzing the signal from the center of k-space acquired during the scan. Since phase-contrast scans are free-breathing, SG should resolve for cardiac and respiratory motion. In this study, we present a robust end-to-end SG algorithm for a novel 2D cine phase-contrast sequence and compared it to the conventional ECG-gating 2D phase-contrast scan.Methods
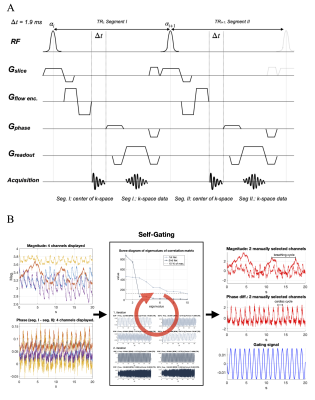
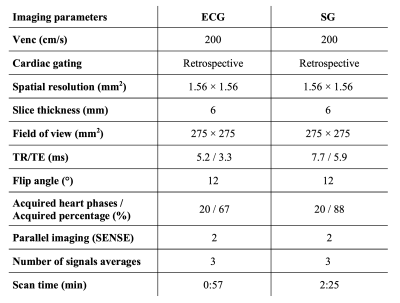
In order to perform SG, we extend the repetition time of an RF-spoiled gradient-echo phase-contrast sequence with a Cartesian trajectory to record the center point of k-space before acquiring the image acquisition (Figure 1A).3 Our SG algorithm augments singular spectrum analysis for advanced reduction of dimensionality to an iterative end-to-end algorithm to ensure complete removal of any adverse motion, gradient dependencies, and noise.4 Pre-processing steps included phase-unwrapping, and removal of high variant coils, that are likely to be corrupted by respiratory motion. After pre-processing, iterative singular spectrum analysis was performed on the reduced number of coils. Our technique combines singular spectrum analysis and power spectral density estimation to select the principal components of the singular spectrum that best resolved the cardiac motion. Only the selected components underwent further iterations until a change in the singular value smaller than a threshold indicated convergence towards a clean gating signal. Finally, the R-R intervals are extracted from the gating signal using a peak detection algorithm based on morphological transformation (Figure 1B). Prior to the retrospective SG, a simulated heart rate was specified for the scan, overestimating the actual cardiac cycle length. To compensate for the excess cardiac cycle length and still achieve the desired temporal resolution within the actual cycle, the overall number of acquired heart phases was increased at the expense of longer scan times. Reconstruction was performed inline on a 1.5T Philips MRI scanner (Achieva d-stream) by implementing our C++ SG library in the Philips reconstruction framework.Two patient studies were conducted to evaluate the performance of SG against the clinical 2D cine phase-contrast sequence with ECG recording. In the first study, the individual R-R intervals detected by the ECG leads and the SG algorithm were compared in 10 patients (3 females, median age 21 yrs.). In the second study, the stroke volume, mean velocity, and peak velocity in the ascending aorta (AO) and the main pulmonary artery (MPA) were measured in 5 patients (1 female, median age 18 yrs.). The imaging parameters for the SG and ECG-gating sequence are shown in Figure 2. All subjects provided informed consent per institution protocol.
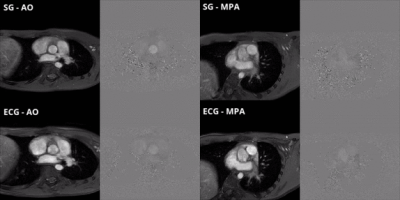
Results
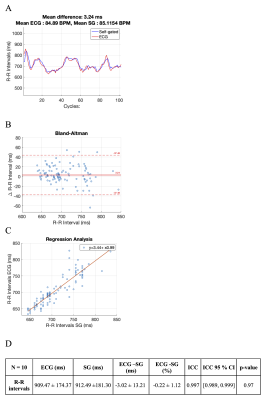
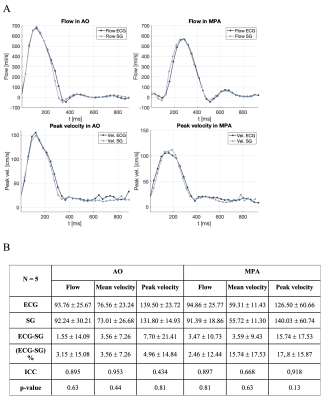
In the first study, patients underwent a clinical cine phase-contrast sequence with ECG leads. There was a <1% error between the estimated R-R intervals by SG and ECG (Figure 3). In the second study, patients completed a free-breathing SG cine phase-contrast sequence without ECG and a clinical free-breathing cine phase-contrast sequence with ECG. ECG and SG scans were reconstructed inline on the scanner and the CVI42 (Circle, Calgary, AB, Canada) software was used for blood flow measurements. There was good to excellent agreement and no significant statistical difference between the blood flow measured at AO and MPA (Figures 4 and 5).Discussion
Lower SNR has been observed in the SG sequence which may be due to the longer echo time associated with the acquisition of the SG signal. While more noise is observed in the static tissue and the lungs of the SG images, there is no perceivable difference in the region of the heart and blood flow measurements. Higher noise levels may be due to the presence of heart rate variability. ECG gated scans accounted for arrhythmia and rejected data acquired at a cardiac cycle that differs by more than 20%, while SG scans included all acquired data.Conclusion
We developed a cardiac SG algorithm for a novel free-breathing 2D cine phase-contrast blood flow measurements and implemented inline reconstruction on the 1.5 T clinical scanner. SG showed comparable measurements and good to excellent agreement with the standard ECG-gating in terms of blood flow and velocity at AO and MPA. Our approach eliminates the need for an ECG signal and allows for the blood flow measurements where an ECG is not accessible such as fetal cardiac MRI.Acknowledgements
This work was supported by a fellowship within the IFI program of the German Academic Exchange Service (DAAD).References
1. D.S. Goolaub, J. Xu, E. Schrauben, L. Sun, C. W. Roy, D. Marini, M. Seed, C. K. Macgowan. Fetal flow quantification in great vessels using motion-corrected radial phase contrast MRI: Comparison with Cartesian. 2020, Journal of Magnetic Resonance Imaging. doi:10.1002/jmri.27334.
2. J. W. Krug and G. Rose. Magnetohydrodynamic distortions of the ECG in different MR scanner configurations. 2011, Computing in Cardiology, pp. 769-772.
3. Y. Zur, M. L. Wood, L. J. Neuringer. Spoiling of transverse magnetization in steady-state sequences. 1991, Magnetic Resonance in Medicine. doi:10.1002/mrm.1910210210.
4. S. Rosenzweig, N. Scholand, H. Christian M. Holme, M. Uecker. Cardiac and respiratory self-gating in radial MRI using an adapted singular spectrum analysis (SSA-FARY). 2020, IEEE Transactions on Medical Imaging. doi:10.1109/TMI.2020.2985994.
Figures