2901
Single-shot compressed sensing versus segmented cine cardiac magnetic resonance in assessing left atrial volume and strain1the First Affiliated Hospital of Nanjing Medical University, Nanjing, China, 2Siemens Healthineers Ltd, Shanghai, China
Synopsis
Left atrial (LA) size is a useful measurement in predicting adverse cardiovascular outcomes. The current study compared LA volume accuracy and strain analysis between conventional segmented cine cardiac magnetic resonance (CMR) and single-shot compressed sensing (CS) cine CMR in 31 and 30 patients with and without left ventricular (LV) diastolic dysfunction, respectively. In both techniques, LA passive ejection fraction and passive radial and longitudinal strain showed diagnostic efficacy for LV diastolic dysfunction, and volume accuracies were not significantly different between techniques. CS cine CMR can reliably assess LA volume and strain in patients who are at risk for cardiovascular diseases.
Introduction
Left atrial (LA) function is closely associated with left ventricular (LV) diastolic function. LA enlargement can predict adverse cardiovascular outcomes and thereby determine patient prognosis and risk stratification 1, 2. Conventional segmented cine cardiac magnetic resonance (CMR) is the gold standard for assessing LA function. However, segmented cine CMR needs multiple breath-holds (BH), which is time consuming and may generate poor image quality due to poor BH and irregular heart rate. Single-shot compressed sensing (CS) cine CMR is a new accelerated technique that can reduce the effects of BH and heart rate 3. CS cine CMR can accurately evaluate LV volume and significantly reduce scanning times 4. The structure and movement between LA and LV are considerably different. This study aimed to compare the accuracy of LA volume and strain analysis between CS and segmented cine CMR.Methods
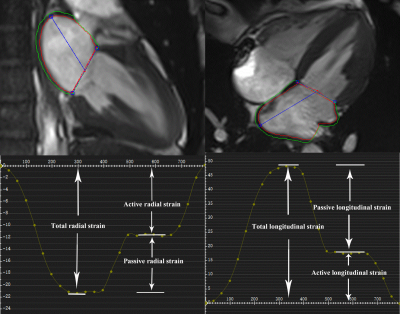
A total of 30 healthy volunteer and 31 patients with LV diastolic dysfunction defined by echocardiography underwent both segmented and CS cine CMR using a 3T MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany). All scans were performed during breath hold after inspiratory. Spatial resolution (1.8*1.6 mm) and slice orientations were the same for the two cine imaging methods. The image quality was evaluated according to a 4-point scale (poor=1, fair=2, good=3, excellent=4).LA volumetric and strain parameters were evaluated by cvi42 software (Circle Cardiovascular Imaging, Calgary, Canada). Based on the biplane area-length method, LA minimal, maximal, and pre-contraction volumes (LAVmin, LAVmax and LAVpre, respectively) were calculated, and corresponding ejection fraction (EF) was generated, including LA total and passive and active EFs. LA strain parameters were from global radial and longitudinal strain curves, which were automatically generated by the software (Figure 1). Both global radial strain and global longitudinal strain contained total, passive, and active strain. Parameters were analyzed independently by two radiologists.
Results
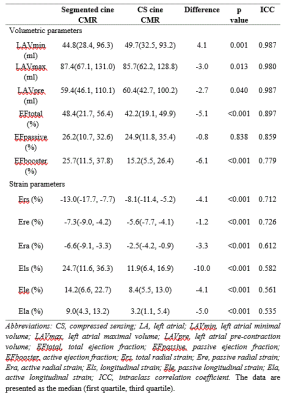
There were no significant differences in image quality between segmented and CS cine CMR (segmented: 3.8±0.4 vs CS: 3.7±0.4, p=0.556). The acquisition duration for CS cine CMR (4s) was considerably shorter than that for segmented cine CMR (28-32s).For both cine methods, there was good to excellent correlation for LA volume and EF (intraclass correlation coefficient [ICC]≥0.779), good correlation for LA radial strains (ICC≥0.612), and moderate correlation for LA longitudinal strain (ICC≥0.535). LA passive EF was not statistically different between methods; however, other volumetric parameters were statistically different. LA radial and longitudinal strain from CS cine CMR were lower (p < 0.001 for all strain parameters) (Table 1).
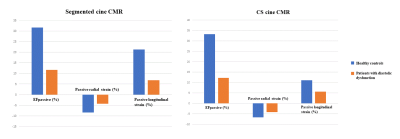
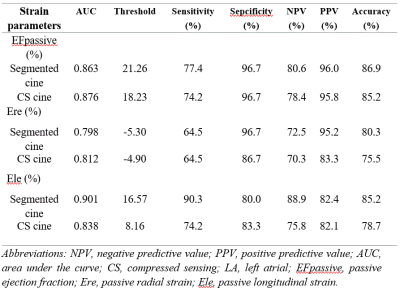
Both CS and segmented cine CMR showed lower LA passive EF and reduced passive radial and longitudinal strains in patients with than without diastolic dysfunction (Figure 2). Specifically, LA passive EF and passive radial and longitudinal strains showed diagnostic efficacy for diastolic dysfunction in both techniques (area under the curve≥0.798) (Table 2). Otherwise, strain parameters did not show diagnostic efficacy for diastolic dysfunction in either technique (p>0.05).
Discussion
In our study, LA passive EF had excellent correlation and similar values between both CMR techniques, which indicates that LA passive EF is interchangeable between techniques. LAVmin was significantly underestimated on CS cine CMR, which may have been caused by unclear mitral valve. The normal references of LA longitudinal strains were various by segmented cine CMR. This finding may be because LA strain is significantly correlated with volumetric indexes, whereas LA volume varies depending on population samples 5.LA strain from CS cine CMR was significantly underestimated, which may be owing to the fact that CS cine CMR presents reduced signal-to-noise ratio and reduced ability to demonstrate the fine structure, and LA blood pool and pericardial fat might have been included in the LA strain analysis. To avoid this, LA myocardial strain analysis should use the same cine CMR method in the future.
At the early phase of LV diastolic dysfunction, reservoir and conduit function decreased and booster pump function was preserved. Impaired LA contractility frequently occurs in patients with severe diastolic dysfunction 6, 7. Our study found patients had significantly impaired LA reservoir, conduit, and booster pump functions detected by both cine CMR techniques, indicating complete LA performance impairment.
Conclusions
Although CS cine CMR may have systematic bias in evaluating LA volume compared to segmented cine CMR, the LA volume and EF still remained clinically comparable between techniques. However, the myocardial strain values from the two techniques should not be interchangeable owing to a remarkable bias between them. LA passive EF and passive radial and longitudinal strain can help diagnose LV diastolic dysfunction using either single-shot CS or traditional segmented cine CMR.Acknowledgements
noneReferences
1. Di Tullio MR, Homma S. Left Atrial Morphology and Function: The Other Side of Cardiovascular Risk. Circ Cardiovasc Imaging. 2016; 9 (2): e004494.
2. Froehlich L, Meyre P, Aeschbacher S, et al. Left atrial dimension and cardiovascular outcomes in patients with and without atrial fibrillation: a systematic review and meta-analysis. Heart. 2019; 105 (24): 1884-91.
3. Yang Y, Yin G, Jiang Y, et al. Quantification of left atrial function in patients with non-obstructive hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking imaging: a feasibility and reproducibility study. J Cardiovasc Magn Reson. 2020; 22 (1): 1.
4. Vermersch M, Longere B, Coisne A, et al. Compressed sensing real-time cine imaging for assessment of ventricular function, volumes and mass in clinical practice. Eur Radiol. 2020; 30 (1): 609-19.
5. Kowallick JT, Kutty S, Edelmann F, et al. Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson. 2014; 16 (1): 60.
6. Reddy YNV, Obokata M, Egbe A, et al. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail. 2019; 21 (7): 891-900.
7. Telles F, Nanayakkara S, Evans S, et al. Impaired left atrial strain predicts abnormal exercise haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail. 2019; 21 (4): 495-505.
Figures