2897
Breath-hold Multi-contrast 3D Dark Blood MRI of the Heart and Great Vessels with Gated Unbalanced T1 Relaxation-Enhanced Steady-State (uT1RESS)1Radiology, NorthShore University HealthSystem, EVANSTON, IL, United States, 2Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 3Radiology, NorthShore University HealthSystem, Evanston, IL, United States, 4Siemens Medical Solutions USA, Chicago, IL, United States, 5Pritzker School of Medicine, University of Chicago, Chicago, IL, United States
Synopsis
We recently described a novel class of steady-state pulse sequence called T1 Relaxation-Enhanced Steady-State (T1RESS). The unbalanced variant, called uT1RESS, generates dark blood images without the need to apply additional preparation modules. We sought to determine whether an electrocardiogram (ECG)-gated implementation of uT1RESS could be used to generate dark blood images for evaluation of the heart and great vessels. A pilot study of healthy volunteers and patients undergoing cardiac MRI was approved by the hospital institutional review board. Initial results suggest that ECG-gated uT1RESS shows promise for volumetric multi-contrast evaluation of the heart and great vessels.
INTRODUCTION
Several pulse sequences are in widespread use for cardiovascular MRI, including spoiled gradient-echo, fast spin-echo (FSE) and balanced steady-state free precession (bSSFP). For dark blood morphological evaluation of the heart, a 2D dual inversion FSE sequence is routinely acquired. Dark blood sequences for T2 mapping and late gadolinium enhancement have also been described. These techniques mostly rely on inflow effects, properly timed inversion pulses or a weak diffusion-sensitized preparation to suppress the blood pool signal (1). We recently described a novel class of steady-state pulse sequence called T1 Relaxation-Enhanced Steady-State (T1RESS) (2), which showed major benefits for imaging of brain tumors. One of the interesting features of the unbalanced variant of this 3D technique, called uT1RESS, is its ability to generate dark blood images without the need to apply additional preparation modules. Dark blood contrast is derived from flow-related dephasing that evolves gradually over the course of the echo train from the use of a 3D unbalanced steady-state free precession readout. Given the high imaging efficiency of the technique, we sought to determine whether a breath-hold, electrocardiogram (ECG)-gated implementation of uT1RESS could be used to generate dark blood images for multi-contrast evaluation of the heart and great vessels.METHODS
A pilot study of healthy volunteers and patients undergoing cardiac MRI for various clinical indications was approved by the hospital institutional review board. Imaging was performed both before and after the administration of gadobutrol (Bayer, Berlin, Germany) on a 1.5 Tesla MRI scanner (MAGNETOM Avanto Dot or MAGNETOM Aera, Siemens Healthcare, Erlangen Germany). A prototype ECG-gated uT1RESS sequence was acquired using a Cartesian 3D unbalanced steady-state free precession readout. The excitation flip angle was approximately 30 degrees, sampling bandwidth was 1838 Hz/px, repetition time was 2.0 msec, and the acquisition window was 162 msec. Parallel acceleration was obtained using GRAPPA with 64 separate reference lines and an acceleration factor of 2, and partial Fourier with a factor of 5/8 was applied along the partition-encoding direction. Spatial resolution was nearly isotropic with voxel dimensions of 1.3 to 1.5-mm after interpolation. Constant flip angle, linear and Kaiser-Bessel preparatory dummy radiofrequency (RF) pulses were tested using from 30 to 100 repetitions. Centric and linear k-space trajectories were compared. Tissue contrast was modified by the incorporation of a magnetization preparation module. For instance, T2-weighted images were acquired by applying a T2 preparation module with echo times of 30 to 90 msec. For imaging of late gadolinium enhancement, a spatially non-selective inversion RF pulse was applied with an inversion time selected based on the TI scout. All scans were acquired in a single breath-hold.RESULTS
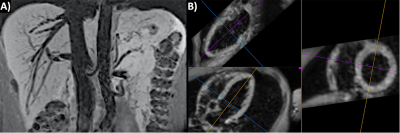
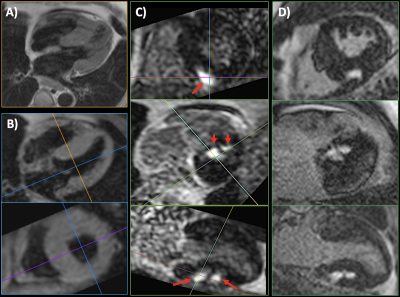
Using ECG-gated uT1RESS, the adequacy of blood pool suppression and image quality were dependent on the k-space ordering, along with the number and flip angle scheme for the preparatory dummy pulses. Optimal image quality and uniform blood pool suppression were obtained using centric k-space ordering in combination with 30 to 60 dummy pulses having a constant flip angle. Applying the dummy pulses with a linear or Kaiser-Bessel flip angle ramp substantially decreased the degree of blood pool suppression. Minimum intensity projections created from breath-hold 3D uT1RESS demonstrated uniform suppression of intravascular signal. Whole-heart 3D dark blood images could be obtained within a single breath-hold either during diastole or at end-systole (Fig. 1), and were able to demonstrate LGE when an inversion preparation was applied (Fig. 2). The image quality of multiplanar reconstructions was excellent. Off-resonance effects were notably absent, and no banding artifacts were seen.DISCUSSION AND CONCLUSION
Our initial results suggest that breath-hold, ECG-gated uT1RESS shows promise for volumetric multi-contrast dark blood evaluation of the heart and great vessels. It utilizes a novel mechanism for the creation of dark blood images, while the very short repetition time steady-state readout and relatively high excitation flip angle provide a better signal-to-noise ratio than can be obtained using a spoiled gradient-echo or fast spin-echo readout. Because centric k-space ordering can be used, the technique is easily combined with a T2 preparation for T2-weighted dark blood imaging, while an inversion preparation can be applied to impart T1 weighting for imaging of LGE. Unlike bSSFP, there are no banding artifacts with the unbalanced steady-state readout. This property may be advantageous for imaging at 3 Tesla or for patients who have a metallic implant such as a pacemaker. Additional work is needed to further optimize the technique and test potential applications such as morphological evaluation of the heart, 3D dark blood imaging of myocardial edema or infarct, and imaging of vessel wall enhancement.Acknowledgements
FUNDING SOURCES: NIH grants R01 HL137920, R01 HL130093 and R01 EB027475.References
1. Henningsson M, et al. JMRI 2020; early view. https://doi.org/10.1002/jmri.27399.
2. Edelman R, et al. Sci Adv. 2020;6(44). Epub 2020/10/30. doi: 10.1126/sciadv.abd1635. PubMed PMID: 33115747.
Figures