2890
A fast navigator (fastNAV) for prospective respiratory motion correction in first-pass myocardial perfusion imaging1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare, Frimley, United Kingdom
Synopsis
A fast respiratory navigator (fastNAV) was developed for dynamic contrast enhanced CMR perfusion imaging by combining spatially non-selective saturation with slice-selective tip-up and slice-selective excitation pulses. A calibration scan was developed to enable the estimation of subject-specific tracking factors. Prospective motion correction using fastNAV was applied to perfusion imaging in 10 patients under free-breathing conditions. Compared to conventional perfusion imaging, fastNAV reduced the effect of respiratory motion while no difference in image quality was observed.
Introduction
First-pass cardiac magnetic resonance (CMR) perfusion imaging is widely used for patients with suspected coronary artery disease. While breath-holding acquisitions are not always effective, prospective respiratory motion correction using a navigator and slice tracking can reduce through-plane motion (1,2). CMR perfusion imaging is based on saturation-recovery, posing a challenge for obtaining a consistent navigator signal while keeping the temporal footprint to a minimum and avoiding a reduction in perfusion image quality (3–7). Furthermore, while a fixed tracking factor is often used to relate right hemi-diaphragm (RHD) navigator motion to cardiac motion (1,2), subject-specific tracking factors can improve motion correction even further (8,9). Here, a fast navigator (fastNAV) was develop for perfusion imaging and tested using subject-specific slice tracking (10).Methods
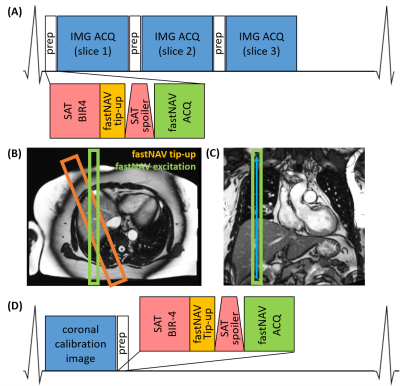
Pulse sequenceThe prototype navigator signal is obtained by combining spatially non-selective saturation (for perfusion imaging) with slice-selective tip-up and slice-selective excitation pulses (Figure 1A&B). The slice-selective tip-up pulse (-90˚flip-angle, 40mm thickness) was between a BIR4-90 adiabatic saturation pulse (11,12) and a spoiler gradient. The navigator excitation slice (15˚ flip-angle, 20mm thickness) was angulated from the tip-up slice in the transverse plane to generate signal from the overlap of these two pulses on the RHD. Gradient-recalled navigator signal was frequency encoded in the superior-inferior direction (Figure 1C). Additional time per navigator: 15ms, including 5ms for real-time feedback.
Subject-specific tracking factor estimation
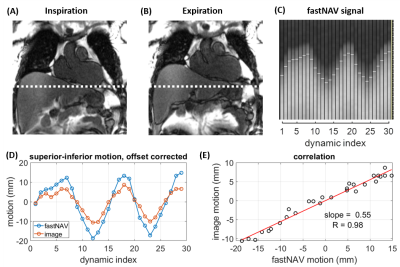
FastNAV-based slice tracking in superioinferior direction was applied to each short-axis slice for perfusion imaging. Conventional cross-correlation analysis (13) was used to extract RHD displacement, which was multiplied by a subject-specific tracking factor. A separate sequence was used to calibrate the subject-specific tracking factor. To allow the near-simultaneous measurement of fastNAV signal and left ventricle (LV) displacement for calibration, one coronal image was acquired per heart-beat, followed by one module including saturation and fastNAV acquisition (Figure 1D). This was repeated 30x to capture several breathing cycles. LV motion was determined offline using a rigid motion estimation algorithm (14). The slope between the LV and fastNAV motion traces was found by least-squares-fitting, which was used as the subject-specific tracking factor, and the correlation coefficient (R) was calculated. Calibration was typically done in 1 minute, once the data was transferred off-line.
Experimental validation
10 Patients were included in this study, which was performed at 1.5T (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). Two dynamic contrast enhanced rest perfusion protocols were performed in each patient, using the fastNAV-based motion correction and a conventional approach without motion correction, with a 10 minute interval in between them to allow for contrast washout. The order of the two perfusion protocols was randomised across subjects. Both perfusion acquisitions used an ECG-triggered balanced steady state free precession (bSSFP) saturation recovery sequence prescribed in the short axis orientation with the following parameters: TR/TE: 2.38/1.04ms, FA:50˚, FOV:360x270mm2, slices:3, slice thickness:8mm, matrix:192x144, voxel size:1.9x1.9mm2, Partial Fourier factor:3/4, GRAPPA:2 (24 integrated reference lines), bandwidth:1085 Hz/pixel, total acquisition duration per heartbeat: 603.9ms, saturation recovery time:133ms, dynamics:60. Patients were instructed to breathe normally.
In-plane motion of the LV was quantified by measuring the average Dice similarity coefficient (15) over dynamics (avDice) as well as the average displacement of the LV center of mass location (avCoM), following manual delineation in each dynamic. Image quality was assessed for each dataset: 4=excellent, 3=minor artefact but not limiting diagnosis, 2=major artefact but not limiting diagnosis, 1=poor image quality and non-diagnostic.
Results
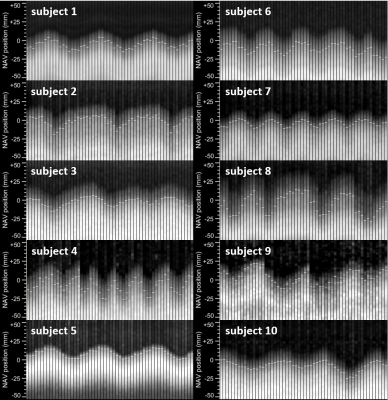
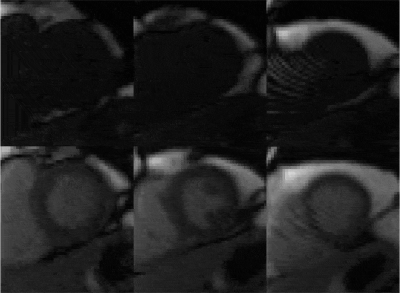
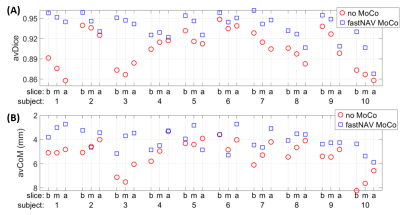
Figure 2 shows an example of tracking factor calibration. Over all patients, subject-specific tracking factors of 0.46±0.13 (min=0.18, max=0.68) were measured with an R value of 0.94±0.03 (min=0.90, max=0.98), indicative of a robust relationship between measured fastNAV and LV motion. A robust fastNAV signal was acquired during perfusion imaging in all patients (Figure 3). An example of motion-corrected perfusion imaging is shown in Figure 4.Images with fastNAV display improved motion metrics in most patient (Figure 5), while overall higher avDice similarity (0.94±0.02 vs. 0.91±0.03, p<0.001) and reduced avCoM displacement (4.03±0.84 vs. 5.22±1.22, p<0.001) were reported. Qualitatively, all slices were scored either as “excellent” or “minor artefact but not limiting diagnosis” with no statistically significant difference overall.
Discussion
FastNAV added only 15 ms to each saturation recovery block, shorter than previously reported navigators for CMR perfusion imaging (3,16,17). The average subject-specific tracking factor across subjects was 0.46, whereas the commonly used factor for conventional RHD NAV (18) is 0.6. The discrepancy might be due to the small sample size in this study.Overall fastNAV-enabled prospective slice tracking resulted in substantial reduction of the global respiratory motion of the heart, but the presence of respiratory-induced local non-rigid deformation as observed in the apical slice of a few patients could not be corrected. The combination of prospective and retrospective motion correction is promising for 3D motion correction.
Future larger studies will be needed to characterise the clinical benefit of fastNAV under stress conditions in patients with coronary artery diseases. The validity of the tracking factor estimated at rest and used during stress conditions remains to be demonstrated.
Conclusion
The fastNAV enabled fast and robust RHD motion tracking in a CMR perfusion sequence. Combined with real-time slice-tracking and subject-specific slice-tracking, fastNAV reduced the effect of respiratory motion.Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (EPSRC) grant (EP/R010935/1), the British Heart Foundation (BHF) (PG/19/11/34243), the Wellcome EPSRC Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z), the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ National Health Service (NHS) Foundation Trust and King’s College London, and Siemens Healthineers. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Danias PG, McConnell M V., Khasgiwala VC, Chuang ML, Edelman RR, Manning WJ. Prospective navigator correction of image position for coronary MR angiography. Radiology 1997;203:733–736 doi: 10.1148/radiology.203.3.9169696.
2. Wang Y, Riederer SJ, Ehman RL. Respiratory Motion of the Heart: Kinematics and the Implications for the Spatial Resolution in Coronary Imaging. Magn. Reson. Med. 1995;33:713–719 doi: 10.1002/mrm.1910330517.
3. Pedersen H, Kelle S, Ringgaard S, et al. Quantification of myocardial perfusion using free-breathing MRI and prospective slice tracking. Magn. Reson. Med. 2009;61:734–738 doi: 10.1002/mrm.21880.
4. Pedersen H, Ringgaard S, Kim WY. A patient-adapted navigator tracking approach for prospective 3D respiratory motion correction in free-breathing myocardial perfusion imaging. In: International Society for Magnetic Resonance in Medicine (ISMRM). ; 2005. p. 512.
5. Bush M, Chen C, Liu Y, Ahmad R, Jin N, Simonetti OP. Cardiac perfusion imaging with prospective respiratory motion correction. In: Proceedings from the 22nd Annual SCMR Scientific Sessions. Belleveu, WA; 2019. pp. 497–499.
6. Basha TA, Roujol S, Kissinger K V., et al. Free-breathing cardiac MR stress perfusion with real-time slice tracking. Magn. Reson. Med. 2014;72:689–698 doi: 10.1002/mrm.24977.
7. Spuentrup E, Manning WJ, Botnar RM, Kissinger K V., Stuber M. Impact of navigator timing on free-breathing submillimeter 3D coronary magnetic resonance angiography. Magn. Reson. Med. 2002;47:196–201 doi: 10.1002/mrm.10026.
8. Moghari MH, Hu P, Kissinger K V., et al. Subject-specific estimation of respiratory navigator tracking factor for free-breathing cardiovascular MR. Magn. Reson. Med. 2012;67:1665–1672 doi: 10.1002/mrm.23158.
9. Bush MA, Ahmad R, Jin N, Liu Y, Simonetti OP. Patient specific prospective respiratory motion correction for efficient, free-breathing cardiovascular MRI. Magn. Reson. Med. 2019;81:3662–3674 doi: 10.1002/mrm.27681.
10. Mooiweer R, Neji R, Sohaib M, Reza N. A fast navigator ( fastNAV ) for prospective respiratory motion correction in first-pass myocardial perfusion imaging. 2020:1–11 doi: 10.1002/mrm.28617.
11. Staewen R, Johnson A, Ross B, Parrish T, Merkle H, Garwood M. 3-D FLASH Imaging Using a Single Surface Coil and a New Adiabatic Pulse, BIR-4. Investig. Radiol. Radiol. 1990;25:559–567 doi: 10.1097/00004424-199005000-00015.
12. Kim D, Cernicanu A, Axel L. B0 and B1-insensitive uniform T1-weighting for quantitative, first-pass myocardial perfusion magnetic resonance imaging. Magn. Reson. Med. 2005;54:1423–1429 doi: 10.1002/mrm.20704.
13. Ehman RL, Felmlee JP. Adaptive technique for high-definition MR imaging of moving structures. Radiology 1989;173:255–263 doi: 10.1148/radiology.173.1.2781017.
14. Roujol S, Benois-Pineau J, De Senneville BD, Ries M, Quesson B, Moonen CTW. Robust real-time-constrained estimation of respiratory motion for interventional MRI on mobile organs. IEEE Trans. Inf. Technol. Biomed. 2012;16:365–374 doi: 10.1109/TITB.2012.2190366.
15. Dice LR. Measures of the Amount of Ecologic Association Between Species. Ecology 1945;26:297–302 doi: 10.2307/1932409.
16. Akçakaya M, Gulaka P, Basha T a, Ngo LH, Manning WJ, Nezafat R. Free-breathing phase contrast MRI with near 100% respiratory navigator efficiency using k-space-dependent respiratory gating. Magn. Reson. Med. 2013;00:1–8 doi: 10.1002/mrm.24874.
17. Cleppien DEJ, Horstick G, Abegunewardene N, et al. Comparison of the quantitative first pass myocardial perfusion MRI with and without prospective slice tracking: Comparison between breath-hold and free-breathing condition. Magn. Reson. Med. 2010;64:1461–1470 doi: 10.1002/mrm.22513.
18. Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020;22:1–18 doi: 10.1186/s12968-020-00607-1.
Figures