2887
Feasibility of motion-resolved high resolution cardiac MRI using a local receiver and MR-tracking micro-coils.1IHU Liryc, Electrophysiology and Heart Modeling Institute, Fondation Bordeaux Université, Pessac, France, 2Univ. Bordeaux, Centre de recherche Cardio-Thoracique de Bordeaux, U1045, Pessac, France, 3INSERM, Centre de recherche Cardio-Thoracique de Bordeaux, U1045, Bordeaux, France, 4Siemens Healthcare SAS, Saint-Denis, France, 5Univ. Bordeaux, Centre de recherche Cardio-Thoracique de Bordeaux, U1045, Bordeaux, France, 6IR4M, UMR8081, Université Paris-Sud/CNRS, Université Paris-Saclay, Orsay, France
Synopsis
In patients presenting cardiac electrical dysfunction, sub-millimetric resolution of CMR would help improving characterisation of the arrhytmogenic substrate. Here, a local coil was placed directly near the anatomic region to image to overpass the lack of sensitivity and selectivity of current conventionnal coils and reduce the voxel size. We implemented a motion compensation method that exploits the signal of micro-coils embodied on a catheter to retrospectively sort radial k-space data prior to ESPIRIT image reconstruction. Using this approach, we present high resolution (300µm), motion-resolved, image of the heart obtained in vivo in pig.
Introduction
Precise 3D characterization of the arrhythmogenic substrate through high resolution MRI would provide new insights for better understanding of electrical dysfunctions in myocardium, such as Atrial Fibrillation (AF). Despite MRI has proven to be valuable for assessing left atrial (LA) myocardial tissue in patients suffering from AF using 3D delayed enhancement (DE-MRI)1, the current spatial resolution (1.5x1.5x2.5 mm3) in clinical scanners remains insufficient since the atrial wall thickness ranges 2 to 5 mm. Limited signal-to-noise ratio (SNR) inherent to large coils surrounding the thorax, together with cardiac and respiratory motions are the main limited factors to improve the image resolution. An intra-cardiac coil would allow a substantial gain in both selectivity (reduced-FOV) and sensitivity (gain in SNR) that in turn may be exploited to improve spatial resolution. It has recently been reported2 that a local coil could achieve high-resolution images (200 µm-in plane) on an ex vivo beating heart. Here, we report on a proof of concept of in vivo high-resolution cardiac images by combining a prototype MRI instrumentation with dedicated image acquisition and reconstruction.Methods
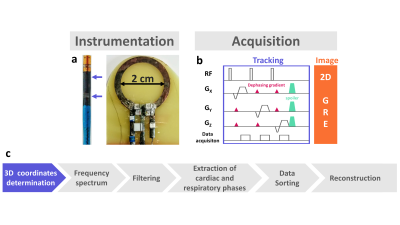
A 2 cm diameter receive-only loop coil was designed using a 35µm-thick copper trace and decoupled from the RF body coil during the transmission. A motion compensation technique3 was implemented that exploits the signal of micro-coils embodied on a MR-compatible catheter (Figure 1a). Determination of the 3D coordinates of the micro-coils with a MR-tracking module4,5 (Figure 1b) was interleaved with acquisitions of N (typically 3 segments) radial golden-angle k-space lines of a 2D gradient-recalled sequence (GRE). Accuracy of the tracking was determined on phantom in static and mobile conditions. In vivo evaluation was performed on a healthy anesthetized pig (protocol approved by the local animal care authorities). After sedation, the animal was intubated and mechanically ventilated (12 breaths/min). Heart rate and respiratory signals were recorded by ECG leads and respiratory belt. An MR-compatible steerable catheter of 9Fr (Vision-MR, Imricor, USA) was inserted through the femoral vein and navigated until the cavity of the right ventricle. The chest of the animal was open and the loop coil was positioned in contact with the epicardium of the left ventricle (LV)..A localization sequence (TruFisp, TR/TE/FA = 381/1.2/80° 1.5 x 1.5x 4.5 cm3) was performed to ensure the loop coil was correctly positioned relative to the LV before high resolution imaging. Acquisition parameters were as follow: FOV = 150 x 150 mm2; Matrix = 448 x 448 px (in-plane resolution of 300 µm); TR/TE/FA = 65/8 ms /65°; bandwidth = 255 Hz/Px; slice thickness = 3 mm; Radial views = 12,000, no respiratory nor cardiac synchronization; 3 segments/TR. Total acquisition time was 4 min 23 s. Tracking parameters were: FOV = 450 mm, matrix = 512 data points, TR/TE/FA = 4.8 ms/2.8 ms/ 3°, bandwidth = 152 Hz/px. After acquisition, k-space data were sorted according to the catheter position after converting signals recorded by the tracking coils into X, Y and Z coordinates (Figure 1c). The frequency spectrum of the direction presenting the dominant amplitude of motion was plotted to visualize peaks corresponding to respiratory and cardiac motions. A Gaussian low pass-filter (sigma = 8) was applied to retrieve the respiratory signals. After subtraction of this filtered data to the initial readings, a 1rst-order Butterworth low-pass filter (normalized cutoff frequency 0.2) was applied to extract displacement related to cardiac contraction. Eleven cardiac phases and 4 respiratory phases were created from these signals. Images were reconstructed for each sub k-space using the ESPIRIT method6.
Results
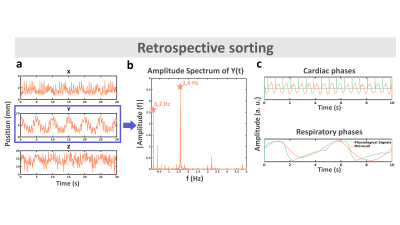
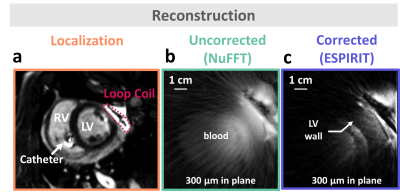
Tracking accuracy was found 0.3 mm on static phantom. Figure 2a displays 3D catheter positions versus time for a 30 sec duration, together with its Fourier spectrum in the Y direction (Figure 2b). Figure 2c displays the respiratory and cardiac signals extracted after processing (orange curves), overlaid on the physiological signals recorded by external sensors (green curves), showing good correspondence. Figure 3a is a scout view of the heart showing the coil and catheter positions. Image in Figure 3b is obtained without data sorting, whereas image Figure 3c is the result of the ESPIRIT reconstruction of 1 cardiac phase and 1 respiratory phase after data sorting. The motion compensation technique drastically improved the image quality when compared to the original one (streaking artifacts are reduced and sharpness of the LV wall is increased).Discussion
We demonstrate the feasibility of obtaining in vivo in pig high-resolution (300 µm in-plane resolution) cardiac images using local 3D motion descriptors (from microcoils) combined with a localized receiver coil to increase SNR. The proposed sequence provides 3D positions of the instrument that is closely linked to physiology, which allows for sorting k-space data prior to iterative image reconstruction. Such an approach opens perspectives for catheter-based imaging (integrating both features in a single deployable instrument) of cardiac muscle with unrivalled spatial resolution to better characterize the cardiac substrate.Conclusion
We were able to combine a local loop coil with a motion compensation algorithm to provide a high resolution image of the left ventricle while overcoming motion artifacts.Acknowledgements
This work was supported by a grant from the French National Research Agency (PRC ANR) (N°ANR-19-CE19-0008-01)
This work was supported by Aquitaine Region (2016 –1R 30113 0000 7550/2016-1R 30113 0000 7553)
This project was supllied by France Life Imaging
References
1. Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and Quantification of Left Atrial Structural Remodeling With Delayed-Enhancement Magnetic Resonance Imaging in Patients With Atrial Fibrillation. Circulation. 2009;119(13):1758-1767.
2. Delcey M, Saniour I, Vaillant F, Abell E, Bour P, Ben Hassen W, Poirier-Quinot M, Quesson B In Proceedings of the 20th Annual Meeting of ISMRM, Sydney, Australia, 2020. Abstract 1196
3. Delcey M, Bour P, Ozenne V, Ben Hassen W, Quesson B In Proceedings of the 20th Annual Meeting of ISMRM, Montreal, Canada, 2019. Abstract 3847
4. Hillenbrand CM, Elgort DR, Wong EY, et al. Active device tracking and high-resolution intravascular MRI using a novel catheter-based, opposed-solenoid phased array coil. Magn Reson Med. 2004;51(4):668-675. doi:10.1002/mrm.20050
5. Homagk A-K, Umathum R, Korn M, et al. An expandable catheter loop coil for intravascular MRI in larger blood vessels. Magn Reson Med. 2010;63(2):517-523.
6. Martin Uecker. Machine Learning Using the BART Toolbox - Implementation of a Deep Convolutional Neural Network for Denoising. Joint Annual Meeting ISMRM-ESMRMB, Paris 2018, In Proc. Intl. Soc. Mag. Reson. Med. 26;2802
Figures