2885
Deep Artifact Suppression For Real Time Phase Contrast Cardiac Magnetic Resonance imaging.1Department of Computer Science, UCL, London, United Kingdom, 2Centre for Cardiovascular Imaging, UCL, London, United Kingdom
Synopsis
Real-time spiral phase contrast MR is practical for free-breathing assessment of flow. However, for sufficient spatial and temporal resolutions it requires high acceleration rates leading to long reconstruction times. Here we propose to train a 3D U-Net with complex convolutions to accurately reconstruct phase data and flow curves from highly undersampled data. Prospectively acquired in-vivo data were reconstructed with similar image quality but ~4.6x faster than compressed sensing reconstructions which could improve workflow.
Introduction
Flow quantification using Phase Contrast Cardiac MR (PCMR) is a vital component of the standard CMR protocol for congenital heart disease. Although most PCMR acquisitions are cardiac gated, real-time methods1,2 have become more common for rapid free-breathing assessment of blood flow. Compressed sensing (CS) has been used to reconstruct high quality images from heavily undersampled real-time spiral PCMR3, but with relatively long reconstruction times. Therefore, we propose a deep learning reconstruction framework to reduce reconstruction times.Methods
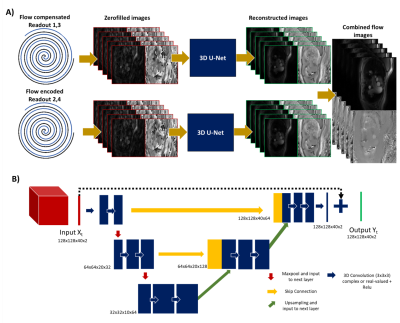
We tested a complex-valued4,5 and two channel real-valued 3D (2D+time) residual U-Nets for deep artefact suppression6 of PCMR data. The network architecture consisted of 3 encoding/decoding blocks with skip connections and two 3D convolution layers (with 32 filters at the initial scale) and 2x2x2 max pool/transpose convolution downsampling/upsampling (Figure 1.B). Training hyper parameters included mean absolute error (MAE) as loss metric, patch size of 128x128x40 (x2 real/imaginary), 50 epochs, an initial learning rate of 0.002, batch size of 2 and an adaptive moment estimation algorithm (Adam)7.The network was trained using a dataset of combined magnitude and phase subtracted images from breath-hold retrospectively gated uniform spiral PCMR data. The dataset included 383 time-series of 40 frames, split into 361/11/11 for training/validation/testing (R-R interval: 0.85±0.18s, temporal resolution: 32.4±3.6 ms). To create paired input images, a smooth random phase was added to the ‘truth’ images (to simulate background offsets seen in uncombined flow-compensated and flow-encoded data), undersampled (using a uniform spiral trajectory), gridded, and cropped (128x128 matrix with 40 frames and 2 flow encoding directions). The 2 different networks (complex and two channel real-valued networks) were compared in simulations using MAE and PSNR.
Additionally, prospective PCMR data was acquired at 1.5T (Aera; Siemens Healthineers AG, Erlangen, Germany) with a real-time spiral GRE sequence (TR/TE=9.75/1.6ms, pixel size= 2.3x 2.3 mm2). The spiral trajectory (R=12) was incremented by the golden angle (~222o) after each pair of flow encoded and compensated readouts was acquired. Flow compensated and encoded images were obtained with temporal resolution of 39 ms, by gridding two spiral readouts for each, performing zero-filling and coil combination using ESPIRiT for coil estimation8 before deep artifact suppression6. The proposed framework is presented in Figure 1.A. In vivo data were qualitatively compared to a CS reconstruction with spatio-temporal total variation regularization (50 iterations of conjugate gradient, 5 iterations using alternating method of multipliers, regularization strength µ=0.005 empirically chosen to balance details vs noise in the reconstruction).
Results
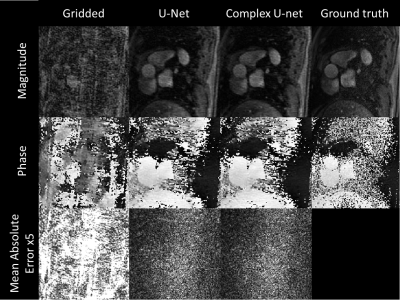
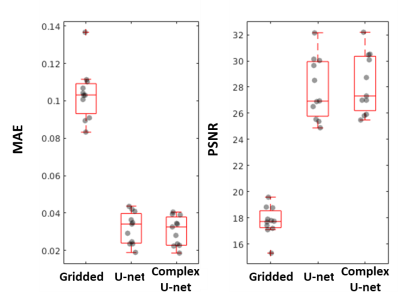
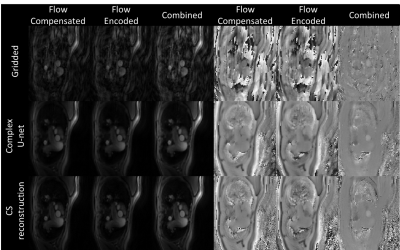
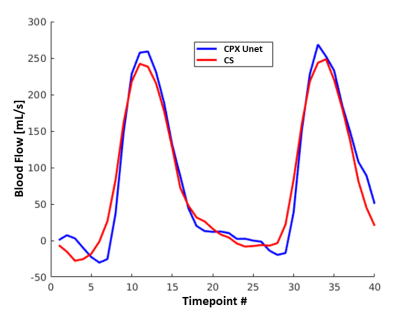
Representative simulated gridded, two-channel U-Net, complex U-Net and ground truth images from the test set are shown in Figure 2. Both networks improved quality from the gridded images with average MAE/PSNR of 0.103/17.75, 0.032/27.86 and 0.030/28.22 for the gridded, U-Net and complex U-Net images with corresponding boxplots in Figure 3. Based on those results the complex-convolution U-Net was used for the prospective in-vivo validation.Figure 4 shows a qualitative comparison of the gridded, proposed and CS imaging results from a prospective patient with congenital heart disease. Reconstruction of 40 frames took 287 s using CS (~7.2s/frame). The proposed method was ~4.6x faster, taking ~62 s (~1.55s/frame) including 2.85s for deep artifact suppression of each encode leading to 5.7 s (~0.14s/frame) of total network inference time and 57 s for gridding (not optimized). Flow curves obtained from CS and complex U-Net are compared in Figure 5 for the same patient.
Discussion
As previously observed4,5, a slight improvement of performance using complex convolutions was observed. The networks were trained on DICOM images only which are largely available in healthcare centers meaning a larger dataset might be used in the future.The ground truth images exhibited some noise (Figure 2) and improvement in the gold standard images could help improve the final images. Additional system imperfections could also be included in simulations to improve the model when applied to real data.
Although reconstructions times were significantly faster for the proposed methods further improvements will be investigated to reduce the preparation time of the gridded images which is now the limiting factor in terms of reconstruction time.
Flow curves between CS and Complex U-Net showed some differences, and further validation against a gold standard acquisition is necessary to understand which provides more accurate clinical metrics.
Conclusion
Deep artifact suppression is proposed for fast reconstruction of real time phase contrast cardiac MR. U-Net with complex convolutions slightly outperformed real valued convolutions. Further investigation to improve the framework and validate the reconstruction in-vivo is necessary.Acknowledgements
This work was supported by the British Heart Foundation (grant: NH/18/1/33511).
References
1. Gatehouse PD, Firmin DN, Collins S, Longmore DB. Real time blood flow imaging by spiral scan phase velocity mapping. Magn. Reson. Med. 1994;31:504–512.
2. Nezafat R, Kellman P, Derbyshire JA, McVeigh ER. Real-time blood flow imaging using autocalibrated spiral sensitivity encoding. Magn. Reson. Med. 2005;54:1557–1561.
3. Kowalik GT, Knight D, Steeden JA, Muthurangu V. Perturbed spiral real‐time phase‐contrast MR with compressive sensing reconstruction for assessment of flow in children. Magn. Reson. Med. 2020;83:2077–2091.
4. Trabelsi C, Bilaniuk O, Zhang Y, et al. Deep Complex Networks. 6th Int. Conf. Learn. Represent. ICLR 2018 - Conf. Track Proc. 2017.
5. Cole EK, Cheng JY, Pauly JM, Vasanawala SS. Analysis of Deep Complex-Valued Convolutional Neural Networks for MRI Reconstruction. 2020.
6. Hauptmann A, Arridge S, Lucka F, Muthurangu V, Steeden JA. Real‐time cardiovascular MR with spatio‐temporal artifact suppression using deep learning–proof of concept in congenital heart disease. Magn. Reson. Med. 2019;81:1143–1156.
7. Kingma DP, Ba JL. Adam: A method for stochastic optimization. In: 3rd International Conference on Learning Representations, ICLR 2015 - Conference Track Proceedings. International Conference on Learning Representations, ICLR; 2015.
8. Uecker M, Lai P, Murphy MJ, et al. ESPIRiT - An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn. Reson. Med. 2014;71:990–1001.
Figures