2884
In Vivo Aortic MR Elastography in Abdominal Aortic Aneurysm Patients: A Potential Biomarker for Predicting Aneurysmal Events1Radiology, The Ohio State University Wexner Medical Center, Columbus, OH, United States, 2Biomedical Engineering, The Ohio State University, Columbus, OH, United States, 3Biomedical Informatics, The Ohio State University Wexner Medical Center, Columbus, OH, United States, 4Surgery, The Ohio State University Wexner Medical Center, Columbus, OH, United States
Synopsis
Abdominal aortic aneurysm (AAA) can result in life-threatening rupture. AAA diameter remains the only clinically useful parameter to predict growth and rupture risk. However, some high-risk AAAs can be overlooked due to their small diameters. AAA stiffness is associated with its extracellular matrix remodeling and thus potentially provides more relevant rupture prediction. Therefore, this study aims to estimate AAA stiffness using aortic MRE in patients with and without aneurysmal events. This is the first study to demonstrate that AAA stiffness and AAA/remote normal (AAA/RN) stiffness ratio are significantly lower in patients with aneurysmal events.
Introduction
The diameter of an abdominal aortic aneurysm (AAA) is generally recognized as a risk factor for its rupture and has been used to warrant elective repairs [1]. Despite its practical significance [2,3], studies have suggested that solely using AAA diameter for rupture risk stratification is not reliable and can lead to delayed intervention of high-risk small AAAs or to unnecessary urgent repairs for the large stable ones [4,5]. Extensive degradation of extracellular matrix (ECM) during the progression of the disease leads to a compromise in mechanical integrity of the AAA wall and eventually to its rupture [6,7]. Aortic stiffness is closely associated with ECM components, making the biomechanical property a more relevant imaging marker for shedding light into AAA rupture potential [8–10].Currently, few techniques are available to non-invasively measure in vivo aortic stiffness. Recently, we have advanced and validated non-invasive in vivo aortic magnetic resonance elastography (MRE) for measuring aneurysm stiffness in an AAA porcine model [5]. Therefore, the goal of this study is to investigate the clinical potential of aortic MRE via a longitudinal study in AAA patients. This work aims to investigate MRE-derived stiffness in stable AAAs and in AAAs that eventually progressed to a large diameter or underwent surgical repair or ruptured.
Methods
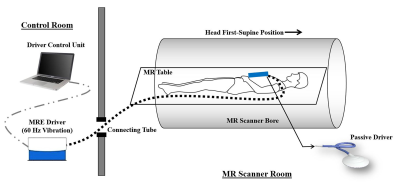
In this study, 73 AAA patients were recruited after approval by the Institutional Review Board (IRB). Sequential aortic MRE follow-ups were performed every 6 months for up to 36 months. Among all patients, 42 patients with both remote normal aorta (RN) and AAA presented for MRE scan for stiffness measures. In these 42 patients, 21 patients had aneurysmal events which include expansion to large diameter (>5.0 cm), surgical repair, or AAA rupture. The non-aneurysmal normal aortic segment between the renal arteries and the infrarenal AAA was defined as the RN segment.In vivo aortic MRE was performed on two 3T MR scanners (Tim Trio and Prisma, Siemens Healthcare, Erlangen, Germany) using an in-house developed rapid GRE MRE sequence [11]. All subjects were scanned in head first-supine position as demonstrated in Figure 1. The imaging parameters included: TE/TR=21ms/25ms. FOV=400x400mm2; reconstruction matrix size=256x256; slice thickness=6mm (50% overlap); No. of slices=3; mechanical excitation frequency=60Hz. A 60Hz flow-compensated (i.e., first-order-gradient-moment-nulled) motion-encoding gradient (MEG) was applied to encode stiffness-modulated shear waves in the aorta.
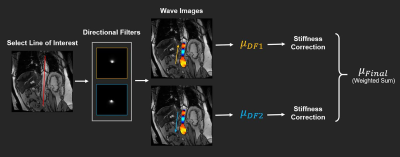
Calculating stiffness in the aorta presents unique challenge due to its limited size, which can lead to biased estimation when using conventional 2D or 3D inversion techniques. Therefore, we propose a new data post-processing routine for estimating aortic stiffness (Figure 2). The proposed method takes advantage of wave information along the axial direction of the aorta extracted by specially designed 4th-order Butterworth directional filters with cutoff 1 to 20 waves/FOV. Finally, an 1D local frequency estimation algorithm is applied on the filtered data for stiffness calculation.
Results and Discussion
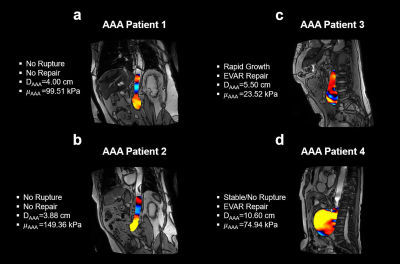
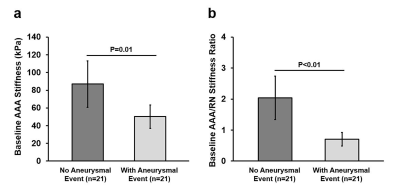
Figure 3 demonstrates MRE wave images and provides stiffness in four AAA Patients. Vascular surgeons were blind to AAA stiffness measurements. Surgical repairs were recommended to the patients based on AAA diameters and growth rate. Lower stiffness was observed in a rapidly expanding AAA when compared to small or stable AAAs.By pooling all 42 patients, AAA stiffness is significantly lower in patients with aneurysmal events than in patients without events (Figure 4a). Significantly lower AAA/remote normal (AAA/RN) stiffness ratio was observed in the patient group with aneurysmal events (P<0.05), suggesting AAA/RN stiffness ratio as a potential biomarker for assessing AAA rupture risk (Figure 4b).
Investigating all patients and their multiple follow-up MRE scans with available AAA stiffness measures, no correlation was observed between AAA stiffness and AAA diameter in patients who experienced aneurysmal events (Spearman ρ=-0.055, P=0.68, n=57) or in those without events (Spearman ρ=-0.127, P=0.32, n=64), suggesting that AAA diameter is a less accurate representation of different wall mechanics altered by the ECM degeneration within a specific patient population.
Conclusion
In the present study, AAA stiffness was successfully quantified using non-invasive and non-contrast-enhanced aortic MRE. The significant difference in AAA stiffness as well as in AAA/RN stiffness ratio between AAAs with and without aneurysmal events emphasizes the relevance of aortic MRE as a potential clinical tool for the management of AAA. Future study will continue to improve the proposed technique and to expand the cohort size through a multi-center collaboration.Acknowledgements
The authors acknowledge clinical coordinator Kristin Thompson.References
1. Brewster DC. Presidential address: What would you do if it were your father? Reflections on endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2001;33(6):1139-1147. doi:10.1067/mva.2001.115374
2. Scott RAP, Ashton HA, Buxton MJ, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: A randomised controlled trial. Lancet. 2002. doi:10.1016/S0140-6736(02)11522-4
3. Powell JT, Brady AR. Detection, Management, and Prospects for the Medical Treatment of Small Abdominal Aortic Aneurysms. Arterioscler Thromb Vasc Biol. 2004;24(2):241-245. doi:10.1161/01.ATV.0000106016.13624.4a
4. Darling RC, Messina CR, Brewster DC, Ottinger LW. Autopsy study of unoperated abdominal aortic aneurysms. The case for early resection. Circulation. 1977.
5. Dong H, Russell DS, Litsky AS, et al. In Vivo Aortic Magnetic Resonance Elastography in Abdominal Aortic Aneurysm. Invest Radiol. 2020;Publish Ah(7):Publish Ahead of Print. doi:10.1097/RLI.0000000000000660
6. Hope TA, Hope MD. Improved risk assessment for abdominal aortic aneurysm rupture: Off-the-wall imaging. J Am Coll Cardiol. 2011;58(24):2531-2532. doi:10.1016/j.jacc.2011.09.016
7. Hellenthal FAMVI, Buurman WA, Wodzig WKWH, Schurink GWH. Biomarkers of AAA progression. Part 1: extracellular matrix degeneration. Nat Rev Cardiol. 2009;6(7):464-474. doi:10.1038/nrcardio.2009.80
8. Vorp DA, Geest JP Vande. Biomechanical Determinants of Abdominal Aortic Aneurysm Rupture. Arterioscler Thromb Vasc Biol. 2005;25(8):1558-1566. doi:10.1161/01.ATV.0000174129.77391.55
9. Wagenseil JE, Mecham RP. Elastin in Large Artery Stiffness and Hypertension. J Cardiovasc Transl Res. 2012;5(3):264-273. doi:10.1007/s12265-012-9349-8
10. Carmo M, Colombo L, Bruno A, et al. Alteration of Elastin, Collagen and their Cross-links in Abdominal Aortic Aneurysms. Eur J Vasc Endovasc Surg. 2002;23(6):543-549. doi:10.1053/ejvs.2002.1620
11. Chamarthi SK, Raterman B, Mazumder R, et al. Rapid acquisition technique for MR elastography of the liver. Magn Reson Imaging. 2014;32(6):679-683. doi:10.1016/j.mri.2014.02.013
Figures