2881
Optimized fast strain-encoding MRI with echo-planar readout1Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Myocardial strain imaging by MRI showed to be an early marker of changes in regional cardiac function before global cardiac function is affected. However, conventional MRI tagging techniques has the limitations of limited spatial resolution and complicated post-processing. Strain-encoding (SENC) showed to be a valuable technique for quantifying myocardial strain with high spatial resolution and simple post-processing. In this study, we provide preliminary results of echo-planar imaging (EPI)-based fast SENC imaging along with imaging artifacts and approaches to minimize their effects. The results showed the feasibility of EPI-based SENC with improved image quality and ultrafast acquisition.
Introduction
MRI is considered the gold-standard for evaluating cardiac function. Recently, myocardial strain imaging by MRI showed to be an early marker of changes in regional cardiac function before global cardiac function, e.g. ejection fraction (EF), is affected. However, conventional MRI tagging techniques has the limitations of limited spatial resolution (determined based on tag spacing) and complicated post-processing. Strain-encoding (SENC) showed to be a valuable technique for quantifying myocardial strain with high spatial resolution (on the pixel level) and simple post-processing that results in color-coded strain maps. Furthermore, using non-Cartesian, e.g. spiral, readout allowed for fast SENC imaging, where a whole series of images throughout the cardiac cycle can be acquired in a single heartbeat of ~1 second. Nevertheless, spiral imaging limitations include undersampling-induced blurring and swirl artifacts and unavailability as a product sequence from some of the major MRI vendors. Echo-planar imaging (EPI) imaging is an appealing alternative for fast data acquisition, which could result in ultrafast readout, which improves temporal resolution, and does not require re-gridding to use conventional fast Fourier Transform (FFT) for image construction. However, EPI has its own limitations, especially off-resonance effects on long readouts. In this study, we provide preliminary results of EPI-based fast SENC imaging along with imaging artifacts and approaches to minimize their effects.Methods
The developed EPI-based SENC technique was tested using a strain phantom compressed by a pneumatic pump and in vivo. A fast-SENC modulation prototype was implemented with a nonselective fat saturation pulse, followed by low- and high-tuning demodulations added to EPI readout. The prototype was implemented and tested on a 3T GE MRI scanner. To be able to run scans without fold-over artifacts and mitigate susceptibility related distortions, a reduced FOV in phase-encoding direction was implemented. Using this two-dimensional (2D) localized SENC, the modulated region was restricted to a cuboid orthogonal to the slice-selection direction. The surrounding non-modulated region thus produces no signal. To be able to preserve signal intensity throughout the cardiac cycle, a variable flip angle acquisition scheme was implemented to compensated for myocardium T1 relaxation. Ramp sampling was implemented to reduce effective echo spacing in EPI readout and mitigate susceptibility issues. Two types of multi-shot acquisitions were implemented: centric and interleaved acquisition strategies. The following imaging parameters were used: myocardium stretch set to 5% and compression set to 30%. For single-shot SENC acquisitions: TE = 8.2 ms, in-plane resolution = 3.8x3.8 mm with 8mm slice thickness. The matrix size was set to 96x76 with 0.8 phase-encoding filed of view. The effective echo spacing was 0.39ms. For multi-shot series, same parameters were used, but the number of shots was set to 2. The effective echo spacing become 0.20 ms. The receiver bandwidth=250kHz by default due to ramp sampling.Results
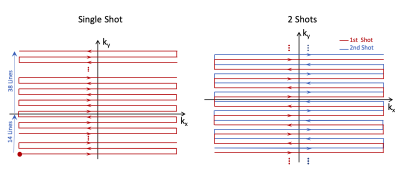
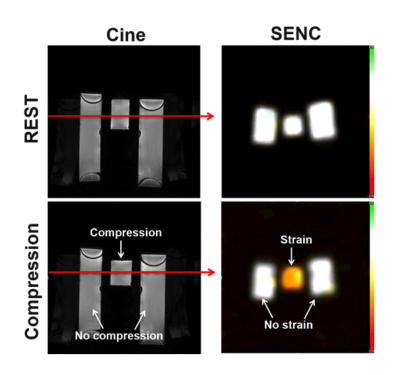
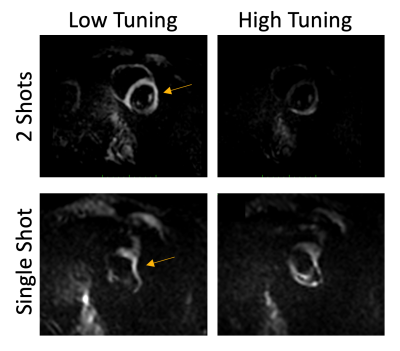
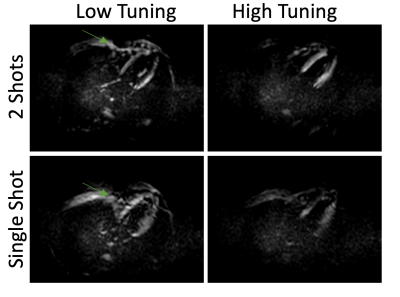
Figure 1 shows different EPI trajectories implemented for SENC acquisition. The phantom experiment (Figure 2) resulted in accurate strain measurement by SENC compared to contraction measured on the cine images. The in vivo results (Figures 3-4) showed off-resonance and geometric distortion artifacts for single-shot acquisition, which were mitigated when switching to 2-shots acquisition, especially with the even-odd interleaved k-space acquisition.Conclusions
EPI-based SENC allows for ultrafast strain imaging with high resolution and accuracy. Special considerations should be adopted when using EPI acquisition to avoid off-resonance artifacts. The developed technique would be valuable for early detection of regional cardiac dysfunction before global function or ejection fraction are affected. The short scan time and no need for breath-holding make the technique cost-effective and patient friendly.Acknowledgements
No acknowledgement found.References
1. Osman et al. Magn Reson Med 46: 324–334.
2. Pan et al. Magn Reson Med 55: 386–395.
3. Ibrahim et al. J Magn Reson Imaging 26: 1461–1470.
4. Ibrahim. Heart Mechanics. Magnetic Resonance Imaging. CRC Press, Boca Raton, FL, 2017.
Figures