2877
Small and Large Respiratory Motions from Free-Running Cardiac Magnetic Resonance Corrected by Two Post-Processing Strategies1Biomedical, Biological & Chemical Engineering, University of Missouri, Columbia, MO, United States, 2Institute for Data Science and Informatics, University of Missouri, Columbia, MO, United States, 3Biochemistry, University of Missouri, Columbia, MO, United States, 4Radiology, University of Missouri, Columbia, MO, United States, 5Biochemistry, Institute for Data Science, University of Missouri, Columbia, MO, United States
Synopsis
Two new methods of automatic, retrospective suppression of breathing motion appear to be effective for real-time cardiac MR (CMR) scans of free-breathing healthy volunteers. This may obviate the need for multiple breath-holds during CMR exams, allowing for a quicker, more comfortable experience for cardiac patients. The first method demonstrated corrects smaller breathing motions and has the advantage of correcting all cardiac cycles. The second method demonstrated successfully compensates the respiratory excursions of largest amplitude, common in short axis scans, by extracting cardiac cycles at end-inspiration or end-expiration.
Background
If cardiac MR (CMR) could be acquired during free breathing with retrospective correction of the breathing motion (1, 2), the exam would be shorter and more comfortable for patients, compared to CINE during breath holds. In real-time CMR, large-amplitude motions at end-inspiration present an especially high hurdle for post-processing to surmount. We developed software to retroactively suppress frame-to-frame heart displacement by respiration, even at end-inspiration.Methods
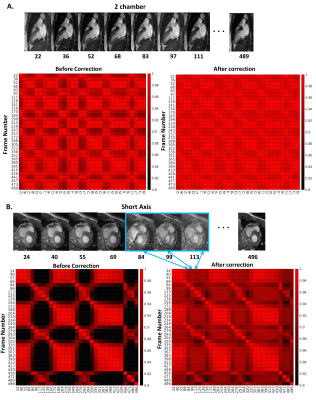
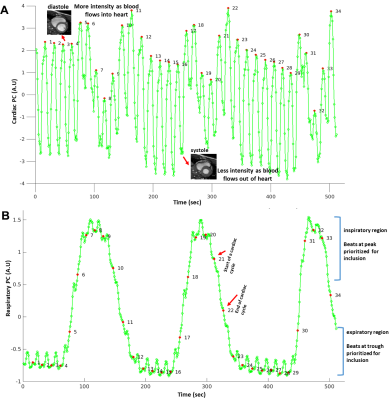
Real-time CMR scans of healthy volunteers were acquired during free-breathing on a 3T Siemens Magnetom Vida scanner using a bSSFP sequence (3–5). Compressed Sensing (6) increased the time resolution of short- and long-axis images to 22 frames/sec (208×170 matrix, voxel size 1.5-1.7×1.5-1.7×6 mm3). Software development began from the TREND package that tracks and resolves multiple concurrent changes between images and spectra using unfold principal component analysis (7). We tailored TrendCMR not only to resolve concurrent cardiac and respiratory motions in dynamic MR scans, but also to make automatic decisions to reconstruct DICOM files with less frame-to-frame displacements of the heart by respiratory motions. Two methods were developed for retrospective removal of breathing motion: Method 1 reconstructs the entire scan with automatic removal of the main breathing motions. Method 2 minimizes the impact of breathing motion by locating and selecting cardiac cycles at end-inspiration or end-expiration and reconstructing separate segments of the scan. Image congruence before and after motion correction was quantified as the correlation coefficient between a frame in systole and the equivalent frame from every other cardiac cycle.Results
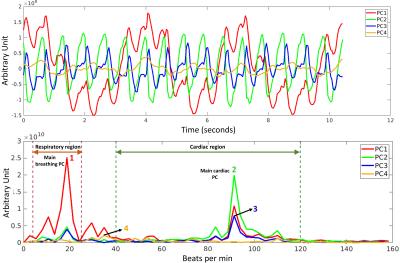
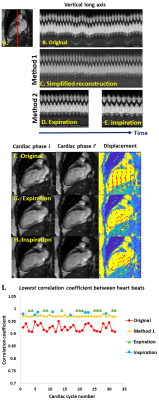
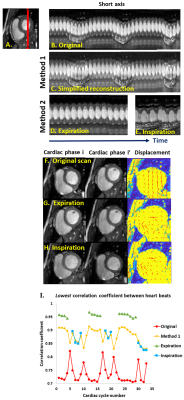
TrendCMR resolved dozens of concurrent motions as principal components (PCs) present in free-running CMR scans of healthy volunteers (Fig. 1A). Power spectra of time courses of the PCs enabled automatic identification of PCs dominated by breathing (Fig. 1B), for omission from reconstructions by Method 1. Method 1 improved the beat-to-beat consistency, making it appealing for correction of entire scans with smaller breathing amplitudes, typical of long axis views, as evident from the improved correlations among beats (Figs. 2, 3C). However, at high-amplitude breathing motions in short axis views, Method 1 introduced ghosts at end-inspiration during systole (Figs. 2B, 4C). To manage high-amplitude excursions, cardiac cycles were grouped by respiratory phase (Method 2). Since there was no triggering during acquisition, each frame’s position within the cardiac cycle was identified retrospectively in the time course of the main cardiac PC, after correcting its sign to make the increased brightness of diastole positive (Fig. 5A). Large breathing motion shifted and decreased apparent amplitude of the cardiac cycle in time courses of the main cardiac PC at end-inspiration (Fig. 5A). At such large and challenging inspirations, an additional automatic search for cardiac cycles was conducted. The heartbeats coinciding with end-expiration in the main respiratory PC (Fig. 5B) were selected for construction of the end-expiratory portion of the scan, and likewise for heartbeats during end-inspiration (Fig. 5B). The excerpts of the original scan obtained by Method 2 retained full cardiac image quality, with suppression of breathing motion that is very good during end-inspiration and excellent during end-expiration (Figs. 3D, 3E, 4D, 4E). While displacement fields of free-running vertical long axis and short axis scans are dominated by respiratory movement of the heart (Figs. 3F, 4F), after correction by Method 2 their displacement fields are dominated by cardiac motions (Figs. 3G, 3H, 4G, 4H). In order to quantify and compare the reproducibility and clarity of cardiac cycles after motion correction by Method 1 or 2, we computed the minimum correlation of each beat with the other beats in that reconstruction, and in the original scan. Method 1 consistently boosted the reproducibility of the beats across the full length of the scan (min. correlation coefficients of 0.90 to 0.965; e.g., Figs. 3I, 4I), with exceptions noted in Fig. 2B, 4C. Method 2 improved the correlation coefficients of inspiratory beats to levels comparable to Method 1 (min. correlation coefficients of 0.92 to 0.985), with sometimes greater clarity. In the case of expiratory beats, Method 2 increased their correlation coefficients, reproducibility, and clarity to minimum correlation coefficients of 0.95 to 0.99 (e.g., Figs. 3I, 4I).Discussion
Here we demonstrate that respiratory motions in free-running CMR can be suppressed by either Method 1 that reconstructs a simplified scan or method 2 that retrospectively navigates to cardiac cycles during end-inspiration and end-expiration. The combination of expedited free-breathing CMR with either method offers the potential to facilitate CMR scanning of patients non-compliant to breath-holding in CINE protocols. This includes patients too frail for multiple breath holds and patients with arrhythmias unsuitable for averaging. Free-running acquisitions combined with retrospective motion correction create the possibility of assessing how cardiac cycles may vary with respiratory phase. To compensate for the limitations of Method 1 during high-amplitude excursions, subjects could be asked to breathe in a shallow manner during short-axis scans.Conclusions
Two methods of retrospective removal of breathing motion in real-time cardiac MRI have been developed and demonstrated. Method 1 removed breathing motion from the entire scan, but during large inspirations introduced ghosts. Method 2 consistently provided sharp images relatively free of respiratory fluctuation, but only during end-inspiration and end-expiration. Users may prefer Method 2 for free-breathing CMR.Acknowledgements
This study was supported by the MU Coulter Biomedical Accelerator Program, and additionally by American Heart Association grant 19IPLOI34760520.References
1. Kellman P, Chefd’hotel C, Lorenz CH, Mancini C, Arai AE, McVeigh ER: Fully automatic, retrospective enhancement of real-time acquired cardiac cine MR images using image-based navigators and respiratory motion-corrected averaging. Magn Reson Med 2008; 59:771–778.
2. Usman M, Atkinson D, Odille F, et al.: Motion corrected compressed sensing for free-breathing dynamic cardiac MRI. Magn Reson Med 2013; 70:504–516.
3. Bieri O, Scheffler K: Fundamentals of balanced steady state free precession MRI. J Magn Reson Imaging 2013; 38:2–11.
4. Chavhan GB, Babyn PS, Jankharia BG, Cheng H-LM, Shroff MM: Steady-State MR Imaging Sequences: Physics, Classification, and Clinical Applications. RadioGraphics 2008; 28:1147–1160.
5. Schär M, Kozerke S, Fischer SE, Boesiger P: Cardiac SSFP imaging at 3 Tesla. Magn Reson Med 2004; 51:799–806.
6. Lustig M, Donoho D, Pauly JM: Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med 2007; 58:1182–1195.
7. Xu J, Van Doren SR: Tracking Equilibrium and Nonequilibrium Shifts in Data with TREND. Biophys J 2017; 112:224–233.
Figures