2870
Real-time cardiac MRI using spiral read-outs and a Variational Network for data-driven reconstruction1Department of Diagnostic and Interventional Radiology, University of Würzburg, Würzburg, Germany, 2Comprehensive Heart Failure Center Würzburg, Würzburg, Germany
Synopsis
Spiral sampling patterns are well suited for fast MR applications like cardiac imaging, yet additional undersampling is necessary to achieve real-time temporal resolution. To still transform these data into images of high quality, a Variational Network is presented. The proposed model uses U-Nets for regularizing a gradient descent scheme, to reconstruct dynamic image series comparable to a segmented fully sampled cine investigation. The acquisition time for an entire stack from base to apex was below one minute; the overall reconstruction time was about 6 minutes.
Purpose
Cardiac functional MRI in clinical routine is usually performed by a segmented acquisition, which requires multiple breath-holds and can fail for arrhythmic patients. To accelerate the data acquisition process towards “real-time” imaging, spiral sampling patterns are well suited, not least because of their incoherent undersampling artifacts.In this work, we implemented a fast spiral gradient echo sequence, which was corrected by a gradient system transfer function (GSTF)-based pre-emphasis to perform cardiac functional imaging in real time. The images were reconstructed by a data-driven Variational Network, which was tailored to process data acquired with non-Cartesian trajectories.
Methods
Spiral readout gradients were implemented in a 2D FLASH sequence for a 3T system (MAGNETOM Prismafit, Siemens Healthcare, Erlangen, Germany) and corrected on-the-fly by a GSTF pre-emphasis1. For every real-time image with a temporal footprint of 50ms, ten subsequently acquired spiral interleaves (TR=5.0ms) were equally distributed in k-space. This represents a moderately undersampled k-space, which is fully sampled in the center, while the FOV constantly decreases towards 44-47mm at kmax. The entire trajectory was rotated for every subsequent image to realize full coverage across several frames. Data was acquired for 8-12 heartbeats. Further measurement parameters were: TE=0.84ms, dwell-time=2.2µs, flip-angle=15°, FOV=480mm×480mm, in-plane spatial resolution=1.34-1.41mm, slice thickness=8mm.To reconstruct the undersampled data, a Variational Network (VN) architecture similar to Hammernik et al.2 and Sriram et al.3 was implemented in the PyTorch framework, comparable to the fastMRI implementation by Zbontar et al.4. To perform fast gridding on GPUs, operators as provided in Muckley et al.5 were used. The model architecture is inherently two-dimensional, processing each time frame separately. As proposed in Hammernik et al.2, the correction of undersampling artifacts is achieved by regarding the reconstruction as an inverse problem to be solved by a regularized gradient descent scheme, where the regularization is provided by data-driven neural networks. The entire model then consists of a fixed number of steps given by the update equation
$$X^{t+1}=X^t-A^*(AX^t-k)+unet(X^t)$$
where $$$k$$$ is the acquired data, $$$X$$$ the reconstructed image and $$$A$$$ denotes the MRI forward operator. As previously proposed3, the regularization was realized by a U-Net model in each step.
Also within the PyTorch framework, the necessary density compensation functions for correcting the non-uniform sampling of k-space were pre-determined by minimizing the mean squared error between a sample k-space and the same k-space subjected to several subsequent applications of the gridding operator and its inverse. Coil sensitivities were obtained from a Nyquist-sampled temporal average using the data of several real-time frames.
The network was trained with undersampled real-time data as input and target images obtained from both a) reconstructions from fully sampled frames using segmented data from several consecutive heartbeats (ECG-gating, breath-hold), and b) compressed sensing (CS) reconstructions of real-time data as presented in Eirich et al.1 (breath-hold and free breathing). In total, this amounted to a training set of 12357 pairs of undersampled real-time frames with target images, obtained from 11 different individuals. The structural similarity index measure (SSIM) was used as loss function and 8 epochs of training were executed on an Nvidia Titan XP GPU.
Results
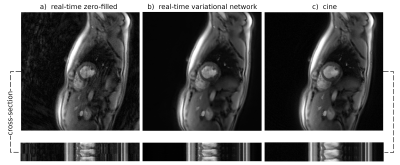
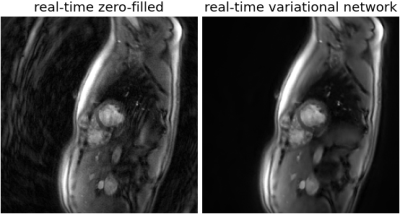
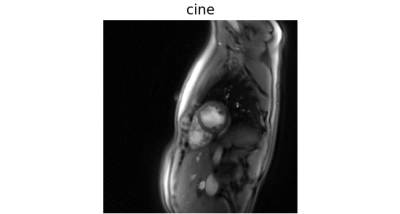
Fig. 1 shows reconstructions of an exemplary mid-ventricular slice in short-axis orientation of one test dataset (previously unseen by the VN). On the left (a), simple gridding of the real-time frames resulted in undersampling artifacts clearly deteriorating the image quality. In the center (b), the reconstruction obtained by applying the suggested VN to the undersampled real-time frames shows no visible artifacts. On the right (c), a frame from a segmented cine in the same cardiac phase is shown, which is of comparable quality as the image in (b). Additionally at the bottom, spatio-temporal depictions of the cross-sections indicated by a dashed line are shown.Fig. 3 shows dynamic real-time data of the same subject, again, both the naïve reconstruction and the output of the VN. To compare this result to the clinical reference standard, Fig. 4 shows a segmented cine of the same slice. The image quality is comparable, however, non-periodic movement like turbulence in the blood flow is blurred in the cine reconstruction but appears sharp in real-time. This is additionally confirmed by the cross-section in Fig. 1.
The VN reconstruction of a series of 20 images (roughly one heart beat) required approximately 30s compared to more than 20min for the according CS reconstruction as presented in Eirich at al.1.
Discussion & Conclusion
The employed VN architecture proved to be well suited for the task of removing undersampling artifacts in spiral MRI data, enabling real-time imaging of the heart at a temporal resolution of 50 ms with high image quality. A clear benefit of this implementation is the short reconstruction time, allowing for reconstruction of all slices from base to apex in about 6min, while the recently proposed CRISPI-implementation1 on a CPU requires several hours. It is noteworthy, that the proposed VN-technique does not apply any model in the temporal domain and hence does not potentially entail any temporal blurring, unlike many of the CS approaches suggested for accelerated dynamic imaging in the past. Higher accelerations and thus higher spatial and/or temporal resolution will be investigated as soon as an adequate amount of training data is available.Acknowledgements
Funding: German Ministry for Education and Research (BMBF), Research Grant 05M20WKAReferences
1. Cardiac real-time MRI using a pre-emphasized spiral acquisition based on the gradient system transfer function, Philipp Eirich et al., MRM 2020
2. Learning a Variational Network for Reconstruction of Accelerated MRI Data, Kerstin Hammernik et al., MRM 2018
3. End-to-End Variational Networks for Accelerated MRI Reconstruction, Anuroop Sriram et al., In: MICCAI 2020, Springer 2020
4. fastMRI: An Open Dataset and Benchmarks for Accelerated MRI, Jure Zbontar et al., ArXiv e-prints 2018
5. TorchKbNufft, a Non-Uniform Fast Fourier Transform for PyTorch, Matthew Muckley, Github Repository, https://github.com/mmuckley/torchkbnufft, 2020
Figures