2863
Automated 3D modeling and analysis of cerebral small vessels with MR angiography at 7 Tesla1State Key Laboratory of Brain and Cognitive Science, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China, 2The Innovation Center of Excellence on Brain Science, Chinese Academy of Sciences, Beijing, China, 3University of Chinese Academy of Sciences, Beijing, China, 4Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China, 5MR Collaboration, Siemens Healthcare Ltd, Beijing, China
Synopsis
Due to the possible interaction between cerebral small vessel disease (CSVD) and a variety of brain diseases or pathological changes, people's interest in small vessel pathology was growing. With the increased imaging resolution at ultra-high field MRI, imaging cerebral small vessels become feasible with TOF-MRA sequence. In this study, we introduced an automated vascular segmentation and tracing method based on deep learning, machine learning and multi filtering. Our method performed well for the multistage branching of cerebral vessels, and could quantitatively evaluate the vasculature. The technique was potentially useful for the clinical studies of CSVD.
Introduction
Time-of-flight MR angiography (TOF-MRA) at 7T has been proved to be a valuable technique for noninvasive imaging of cerebral small vessels, especially for lenticulostriate artery (LSA)1. However, the accurate extraction of the vasculature of the cerebral small vessels remains challenging, due to the resolution and artifact of MRI image. Minimal path algorithm was proposed to partially solve the problem23. Recently, with the development of deep learning, convolutional neural network is expected to be a better solution to this problem4. In this work, we realized the automated extraction of lenticulostriate arteries (LSA) from 7T TOF-MRA images by convolutional neural network (CNN) and random forest method. Quantitative features of LSA was also obtained.Methods
The images of 5 subjects were used as training data, while 5 other images were used for testing. The images were acquired on a 7T research MRI system (Siemens, Erlangen, Germany). The imaging protocol was resolution = 0.23×0.23×0.34 mm3, matrix = 576×768×144, TR = 15ms, TE = 3.57ms, FA = 20°.In the preprocessing, brain extraction and inhomogeneity correction were done by FSL5 and N4 bias field correction6 on Python, respectively. The segmentation algorithm was shown in Algorithm 1. Large arteries (such as middle cerebral arteries) were segmented by intensity thresholding.
The CNN was trained for the segmentation of small vessels. There were 1600 positive patches and 12000 negative patches were used as the training unit for high robustness. The scale of patch is 11 × 11 × 11. After the vessel mask was generated, we used the mean filter to remove the scattered false positive voxels and smooth the edge of the vessels. In the tracing stage, the most appropriate cylinder vertex position was determined by the random forest method, which had the following features:
a. The number of blood vessel points in the cylinder.
b. The variance of the distance to the edge of the blood vessel.
c. The vector deflection angle determined in this calculation.
d. The number of blood vessel points around the target point.
e. The Gaussian distribution weight of brightness.
According to the above characteristics, the position of the next point of the cylinder was determined comprehensively. At the same time, we tolerated a certain degree of vascular disruption. When the tracing ends, Bezier spline curve was built to represent the centerline of cerebral small vessels. According to the radius of the cylinder in each step, we found the branch points in the spherical region which is n times larger than the radius, and added them to the candidate points that prepare to screen. The method was shown in Algorithm 2.
Results
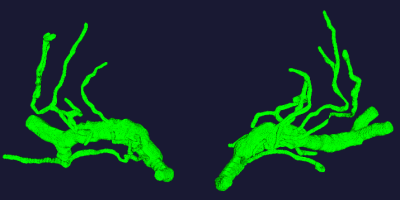
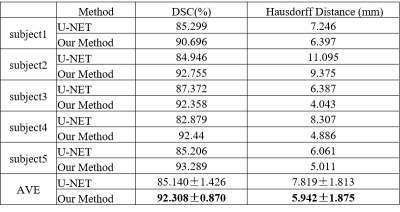
A representative vascular model was shown in Figure 1. Compared with U-NET, our method achieved higher dice similarity coefficient (DSC) and lower Hausdorff Distance. Especially, in the area of small vessels, our method demonstrated better segmentation of the vasculature. Table 1 and Figure 2 showed the comparison results.In 5 images, the number of LSA stems was found to be 7.60±0.10 , while the number of branches were 11.40±0.10. The length is 27.34±5.45 mm, the radius is 0.25±0.03 mm.
Discussion
The main contributions of this study are that, in TOF-MRI images, we combine CNN for segmentation and random forest for vascular tracing. This idea excludes the false positive results while shows the complex structure of small blood vessels. It ensures a high true positive rate of revascularization. In the random forest algorithm, five effective features are defined to determine the next step in the tracking process. These features only need local information and therefore are relatively cost lower. There are still some limitations in our research. The false positives mainly come from the pulsation artifacts produced by the pulsation of large arteries. The procedure that specifically remove such kind of artifacts can be added between segmentation and tracing. On the other hand, as the cone search in tracing process is discrete, there is a certain discretization error which leads to the center line offset.Conclusions
We proposed a comprehensive way for the segmentation and tracking of cerebral small vessels from 7T TOF-MRA images. The accuracy of vascular modeling was improved compared with previously proposed algorithm.Acknowledgements
This work was supported by the National Natural Science Foundation of China (82001804, 8191101305), the Natural Science Foundation of Beijing Municipality (7191003), the National Key Research and Development of China (2017YFC1307904), the capital health research and development of special (2020-2-5115), and the Strategic Priority Research Program of Chinese Academy of Science (XDB32010300).References
1. Grochowski, C. and G. Staśkiewicz, Ultra high field TOF-MRA: A method to visualize small cerebral vessels. 7T TOF-MRA sequence parameters on different MRI scanners – Literature review. Neurologia i Neurochirurgia Polska, 2017. 51(5): p. 411-418.
2. R. Phellan, A. Peixinho, A. Falcão , et al. Vascular Segmentation in TOF MRA Images of the Brain Using a Deep Convolutional Neural Network. 2017. Cham: Springer International Publishing.
3. Schoenemann, T., S. Masnou, and D. Cremers, et al. The Elastic Ratio: Introducing Curvature Into Ratio-Based Image Segmentation. IEEE Transactions on Image Processing, 2011. 20(9): p. 2565-2581.
4. ZF. Zhao, Y. Chen, Y. Hou, et al., Segmentation of blood vessels using rule-based and machine-learning-based methods: a review. Multimedia Systems, 2019. 25(2): p. 109-118.
5. V. Popescu, M. Battaglini, W. S. Hoogstrate, et al., Optimizing parameter choice for FSL-Brain Extraction Tool (BET) on 3D T1 images in multiple sclerosis. NeuroImage, 2012. 61(4): p. 1484-1494.
6. R. G. Boyes, J. L. Gunter, C. Frost, et al., Intensity non-uniformity correction using N3 on 3-T scanners with multichannel phased array coils. NeuroImage, 2008. 39(4): p. 1752-1762.
Figures

Algorithm 1. Vessel segmentation