2855
Neurovascular coupling in the cerebellum: reconstructing the neurophysiological basis of different cerebellar fMRI responses.1Brain Connectivity Center Research Department, IRCCS Mondino Foundation, Pavia, Italy, 2Brain and Behavioral Sciences, University of Pavia, Pavia, Italy, 3Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Faculty of Brain Sciences, University College London, London, United Kingdom
Synopsis
Neuroimaging studies in humans showed different patterns of BOLD responses in cerebellar vermis and cortical regions when performing tasks at different grip forces. In this study we investigated the basis of different cerebellar fMRI responses ex-vivo. Capillary dilation and neuronal activity were recorded in the vermis and hemisphere of mouse cerebellar slices, highlighting a region and frequency dependency of neurovascular responses in the granular layer. The correlation between neuronal activity and vessel diameter changes was explored through a computational model, which supported a central role played by the NMDAR-NO pathway in shaping the vasodilation time course.
Introduction
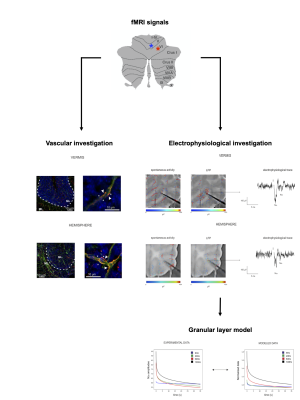
In humans, fMRI investigations showed linear and non-linear blood-oxygen-level-dependent (BOLD) responses to variable grip forces in the cerebellar vermis and hemisphere during the execution of a motor task1,2. In order to understand the possible source of such non-linear behavior, we investigated neurovascular coupling (NVC) mechanisms ex-vivo, reconstructing capillaries motility and their neuronal correlates in cerebellar acute slices. Moreover, we explored the correlation between neuronal activity and vessel diameter changes performing a biophysical realistic reconstruction of network activity through computational models (Fig.1), proposing a possible explanation for in-vivo in humans BOLD observations.Methods
Experimental setting: sagittal acute slices were obtained as previously described3 from cerebellar vermis and hemisphere of C57BL/6 mice. Recordings of vessel dynamics and neuronal responses to mossy fiber stimulation were performed in the granular layer (GL), given its role in determining cerebellar NVC3. Mossy fibers were stimulated for 35 seconds at different frequencies (6Hz,20Hz,50Hz,100Hz) in order to mimic different ranges of activity. Statistical significance was assessed using Student’s t test.Vascular investigation: Time-lapse recordings of vessel caliber in the GL were obtained using bright-field microscopy, identifying capillaries through their diameter and the presence of pericytes3. Capillaries changes were quantified using the ImageJ Software4.
Electrophysiological investigation: Neuronal responses to mossy fiber stimulation were recorded as Local Field Potential (LFP)(N2a and N2b peaks5) in the GL, using a high-density multielectrode array (HD-MEA;Biocam X,3BrainAG), which enables high spatial and temporal resolution, with 4096 electrodes (42µm inter-electrode distance) recording simultaneously. LFP signals were analyzed with ad-hoc routines written in MATLAB (Mathworks). The analysis of GL LFPs was focused on N2a and N2b peak amplitudes, as measures of postsynaptic granule cells synaptic activation5. The cumulative integral of the LFP peaks during the 35 seconds of stimulation was computed as a tentative measure of total neuronal activity6.
Granular layer model: A detailed and biophysically realistic model of the GL7 in the vermis was used to dissect the neuronal response from the experimental LFP. In the simulation, about 50 contiguous glomeruli were stimulated reproducing the experimental setting. Granule cells spiking activity and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor8 mediated synaptic currents were extracted to test the correspondence between simulated and electrophysiological data. Currents derived from N-methyl-D-aspartate (NMDA) receptors8 activation were extracted from the model and used to compare neuronal activity and vessel dynamics in the NVC of the GL.
Results
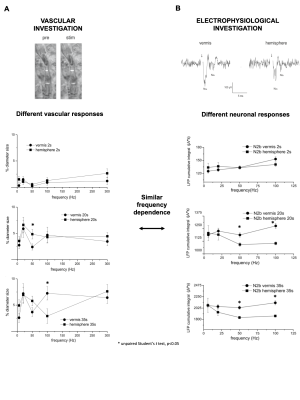
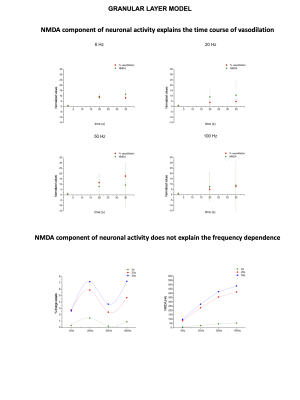
Significant capillary dilation was recorded both in the vermis and hemisphere cerebellar slices in response to the different frequencies of mossy fibers’ activation. Interestingly, the NVC response did not increase linearly with the input frequency, showing different non-linear responses in the vermis compared to the hemisphere (Fig.2A). After a single pulse stimulation of mossy fibers LFP signals appeared to be different in terms of peaks amplitudes between the vermis and the hemisphere. Interestingly, unlike the N2a, the N2b component showed a non-linear trend at increasing frequencies, suggesting it might play a role in non-linear vessel responses (Fig.2B). Since the N2b peak is affected by NMDA receptors, a theoretical model was applied to isolate the NMDA receptor-mediated component of the GL cell responses.High correlation values were found between the GL responses in the model and in the experimental electrophysiological data at different input frequencies (R2>0.9), supporting the validity of our biophysically realistic model to reconstruct GL activation. Interestingly, the dynamics of NMDA currents during the stimulation matched the trend in the time-course of vessel caliber changes. On the other hand, NMDA currents did not account for the non-linear frequency-dependence of capillaries vasodilation (Fig.3). Computational model adjustments are under development in order to investigate the NVC mechanisms in the cerebellar hemisphere.Discussion
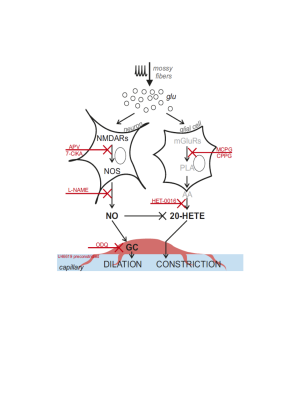
Our results show for the first time that NVC in the cerebellum GL has a non-linear frequency-dependence, resulting in different capillary dilation depending on the mossy fibers activation frequency. Moreover, the frequency-dependence of the capillary vasodilation was not identical in the cerebellar vermis and hemisphere. To investigate the origin of this phenomenon, the GL neuronal activity was measured and then dissected using a computational model of the GL validated against experimental data. NMDA currents explained the time course of the capillary vasodilation during stimulation, in agreement with the key role of NMDA receptors-dependent Nitric Oxide production regulating NVC in the GL3(Fig.4). On the other hand, the NMDA component was not sufficient to explain the non-linearity observed in vessel responses to different input frequencies, suggesting the involvement of additional mechanisms determining the frequency-dependency of NVC. A plausible hypothesis could involve a competition between vasodilation and vasoconstriction pathways, relying on NMDA and metabotropic glutamate receptors activities, whose different activation properties might account for the frequency-dependency. This scenario is supported by the previously reported interplay between vasodilation and vasoconstriction biochemical pathways in the cerebellum3,9(Fig.4). Taken together, our results provide a novel approach to dissect the NVC mechanisms ex vivo and, most importantly, support a frequency-dependency of the NVC in the cerebellum. To the best of our knowledge, this is the first investigation of frequency-dependency of the NVC in physiological conditions. Moreover, these data support the region dependency of neurovascular mechanisms in the cerebellum, ultimately providing a neurophysiological substrate to the non-linearity observed during fMRI recordings in humans.Acknowledgements
This research received funding by H2020 Research and Innovation Action Grants Human Brain Project 785907 and 945539 (SGA2 and SGA3) and by the MNL Project “Local Neuronal Microcircuits” of the Centro Fermi (Rome, Italy) to ED and FP. CGWK received funding from the UK MS Society (#77), Wings for Life (#169111), Horizon2020 (CDS-QUAMRI, #634541), BRC (#BRC704/CAP/CGW).References
1. Alahmadi, A. A., M. Pardini, R. S. Samson, K. J. Friston, A. T. Toosy, E. D'Angelo & C. A. Gandini Wheeler-Kingshott (2017) Cerebellar lobules and dentate nuclei mirror cortical force-related-BOLD responses: Beyond all (linear) expectations. Hum Brain Mapp, 38, 2566-2579.
2. Casiraghi, L., A. A. S. Alahmadi, A. Monteverdi, F. Palesi, G. Castellazzi, G. Savini, K. Friston, C. A. M. Gandini Wheeler-Kingshott & E. D'Angelo (2019) I See Your Effort: Force-Related BOLD Effects in an Extended Action Execution-Observation Network Involving the Cerebellum. Cereb Cortex, 29, 1351-1368.
3. Mapelli, L., G. Gagliano, T. Soda, U. Laforenza, F. Moccia & E. U. D'Angelo (2017) Granular Layer Neurons Control Cerebellar Neurovascular Coupling Through an NMDA Receptor/NO-Dependent System. J Neurosci, 37, 1340-1351.
4. Schneider CA, Rasband WS, Eliceiri KW. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods. Jul;9(7):671-5. doi: 10.1038/nmeth.2089.
5. Mapelli, J. & E. D'Angelo (2007) The spatial organization of long-term synaptic plasticity at the input stage of cerebellum. J Neurosci, 27, 1285-96.
6. Mathiesen, C., K. Caesar & M. Lauritzen (2000) Temporal coupling between neuronal activity and blood flow in rat cerebellar cortex as indicated by field potential analysis. J Physiol, 523 Pt 1, 235-46.
7. Casali, S., M. Tognolina, E. D'Angelo (2020) Cellular resolution mapping uncovers spatial adaptive filtering at the cerebellum input stage. bioRxiv 2020.03.14.991794
8. E.R., K. 2003. Principles of Neural Science. CEA.
9. Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468:232-243.
Figures