2853
Realistic diffusion tensor cardiovascular magnetic resonance simulations in a histology-based substrate: The effect of membrane permeability1Department of Aeronautics, Imperial College London, London, United Kingdom, 2Cardiovascular Magnetic Resonance unit, Royal Brompton Hospital, London, United Kingdom, 3National Heart and Lung Institute, Imperial College London, London, United Kingdom
Synopsis
Computational simulations and specifically random walks provide a unique opportunity to investigate how several confounding factors might affect the diffusion tensor cardiovascular magnetic resonance (DT-CMR) parameters. We investigate the effect of membrane permeability and diffusion time on the fractional anisotropy (FA) and mean diffusivity in a realistic histology-based 3D substrate considering two common sequence types. Comparing the results from permeable with that from impermeable membranes helps to considerably reduce FA towards levels expected from in-vivo DT-CMR. We further reveal that FA is unaffected by changes in diffusion times above 500ms. A similar, albeit weaker effect is observed regarding MD.
Introduction
Diffusion tensor cardiovascular magnetic resonance (DT-CMR) provides non-invasive measures of cardiac microstructure, but linking changes in DT-CMR results to specific changes in the cardiac microstructure is challenging. Biological tissue is generally composed of several complex elements which might be modelled as compartments with differing intra-compartmental diffusivities separated by semi-permeable membranes. Numerical simulations offer the opportunity to study the effects of changes in microstructure or in acquisition parameters without confounding effects. In our previous work [1], we performed simulations in a realistic 3D virtual microstructure based on porcine heart histology. We assumed impermeable membranes but found that fractional anisotropy (FA) was overestimated compared to in-vivo studies. Recent work using a finite difference simulation [2] suggests that including permeable membranes reduces the observed FA.In this work we use the virtual tissue detailed in [1] and implement an existing method [3] (transit model) that incorporates membrane permeability in the random walk simulations and allows for differing diffusivities between compartments. We investigate how membrane permeabilities within the expected range in cardiomyocytes modifies the DT-CMR parameters FA and mean diffusivity (MD) using two commonly used DT-CMR sequences, pulse gradient spin echo (PGSE) and stimulated echo acquisition mode (STEAM).
Methods
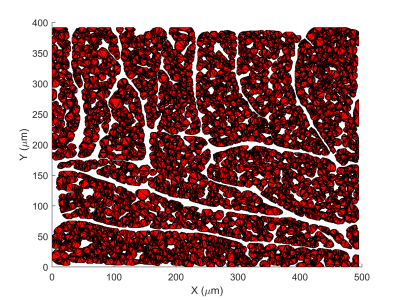
We perform Monte Carlo random walk simulations using a method employed in previous work [1]. As shown in Figure 1, we select a substrate with an extra-cellular volume fraction (ECV) of approximately 25% and set intra-cellular (IC) and extra-cellular (EC) diffusivities to $$$D_\text{ICS}$$$=1.5 and $$$D_\text{ECS}$$$=3µm2/ms respectively. Simulations are performed in a 2.8x2.8x8mm3 voxel with 104 walkers and a constant step size based on the maximum probability restriction suggested in [3]. We consider two pulse sequences matching the parameters published in [1]: one short diffusion time ($$$\Delta$$$=20.5ms) spin-echo sequence (PGSE) and a STEAM sequence ($$$\Delta$$$=1000ms), each with a gradient strength of approximately 40mT/m per axis and a b-value of 450μm2/ms. The membrane permeability is modelled according to [3], which gives a probability of transit $$p_t = \frac{2 \kappa \mathrm{d}s}{D + 2 \kappa \mathrm{d}s}$$, where $$$D$$$ is the diffusivity of the compartment the walker is attempting to exit, $$$\kappa$$$ is the membrane permeability and $$$\mathrm{d}s$$$ is the normal distance from the origin of the step to the intersection with the membrane.Two studies are carried out: first, we investigate the effect of varying membrane permeability on common DT-CMR pulse sequences; then, we demonstrate how the effect of permeability changes as the diffusion time ($$$\Delta$$$) increases in the STEAM sequence by comparing a feasible biological permeability with the impermeable case.
Results
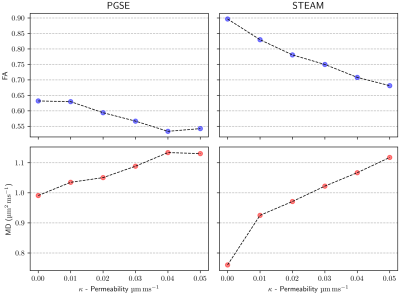
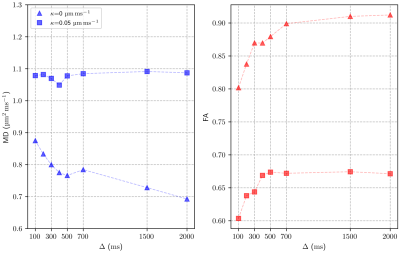
Figure 2 shows fractional anisotropy (FA) and mean diffusivity (MD) values for a range of membrane permeabilities ($$$\kappa$$$=0-0.05μm/ms). The results obtained for the impermeable case match those obtained in the simulations performed in [1]. We observe that the FA decreases almost linearly with increasing membrane permeability $$$\kappa$$$, while MD increases (again, almost linearly) with $$$\kappa$$$.Figure 3 compares two extreme permeability cases ($$$\kappa$$$=0 and 0.05μm/ms). We study the effect of diffusion time ($$$\Delta$$$) on FA and MD in a STEAM sequence and observe significant variations of FA and MD for diffusion times $$$\Delta$$$=100-700ms. Beyond 700ms, FA does not change significantly. Similar behaviour is observed for both the permeable and impermeable cases, however, the highly permeable membranes seem to limit the MD reduction over time and rapidly reach a steady state.
Discussion
The FA measures the degree of anisotropy of the diffusion of walkers as observed by DT-CMR. Diffusion times of the order of 1s are frequently used in DT-CMR to minimise cardiac motion effects and probe cardiac microstructure on longer length scales than are available using spin-echo sequences. The pencil-like shape of cardiomyocytes is fully probed by the walkers during this long time and this leads to an increase in FA compared to a short PGSE sequence. Figures 2 and 3 show that permeability of the cell membrane reduces the anisotropy of the measured DT-CMR signal and increases the distances diffused as the random walkers cross multiple compartment boundaries. Even for the short diffusion times used in PGSE the increase of FA and reduction of MD with increasing membrane permeability is remarkable.While including membrane permeability within our model drives our FA values towards those observed in-vivo [4], FA is still over-estimated. In future studies we will investigate more realistic virtual microstructures, the effects of perfusion and the limitations of the transit model to resolve this discrepancy.
Conclusions
Several studies have shown the utility of DT-CMR in providing insights into myocardial microstructure in disease [6]. We show the effect of permeability in two commonly used sequences: STEAM and PGSE. We conclude that cell membrane permeability reduces the restriction of diffusion, which is reflected in a reduction in FA and an increase in MD. Incorporating membrane permeability into random walk simulations of DT-CMR makes the simulations more realistic. Future work might involve improving the transit model step restriction or studying the impact of permeability analysing walker migrations between extra-cellular and intra-cellular space.Acknowledgements
This work was funded by British Heart Foundation Grant RG/19/1/34160.References
[1] Rose JN, Nielles-Vallespin S, Ferreira PF, Firmin DN, Scott AD, Doorly DJ. Novel insights into in‐vivo diffusion tensor cardiovascular magnetic resonance using computational modelling and a histology‐based virtual microstructure. Magnetic Resonance in Medicine. 2019. DOI:10.1002/mrm.27561
[2] Rose JN, Sliwinski L, Nielles-Vallespin S, Scott AD, Doorly DJ. Studying the effect of membrane permeability with a GPU-based Bloch–Torrey simulator. Proceedings of ISMRM 2019.
[3] Fieremans E, Novikov DS, Jensen JH, Helpern JA. Monte Carlo study of a two-compartment exchange model of diffusion. NRM in Biomedicine. 2010. DOI:10.1002/nbm.1577
[4] Scott AD, Nielles-Vallespin S, Ferreira PF, Khalique Z, Gatehouse PD, Kilner P, Pennell DJ, Firmin DN. An in-vivo comparison of stimulated-echo and motion compensated spin-echo sequences for 3 T diffusion tensor cardiovascular magnetic resonance at multiple cardiac phases. J Cardiovasc Magn Reson. 2018. DOI:10.1186/s12968-017-0425-8
[5] Nielles-Vallespin S, Mekkaoui C, Gatehouse P, Reese TG, Keegan J, Ferreira PF, Collins S, Speier P, Feiweier T, de Silva R, Jackowski MP, Pennell DJ, Sosnovik DE, Firmin D. In vivo diffusion tensor MRI of the human heart: reproducibility of breath-hold and navigator-based approaches. Magnetic Resonance in Medicine. 2013. DOI: 10.1002/mrm.24488
[6] Ming-Ting Wu, Mao-Yuan M. Su, Yi-Luan Huang, Kuan-Rau Chiou, Pinchen Yang, Huay-Ben Pan, Timothy G. Reese, Van J. Wedeen, Wen-Yih I. Tseng. Sequential Changes of Myocardial Microstructure in Patients Postmyocardial Infarction by Diffusion-Tensor Cardiac MR Correlation With Left Ventricular Structure and Function. Circulation: Cardiovascular Imaging. 2009. DOI:10.1161/CIRCIMAGING.108.778902
Figures