2826
Impact of the Acquisition Protocol on the Sensitivity to Demyelination and Axonal Loss of DKI and NODDI: A Simulation Study1Department of Neuroscience, Imaging, and Clinical Sciences, University of Chieti Pescara G. D'Annunzio, Chieti, Italy
Synopsis
There is a general lack of agreed-upon guidelines regarding the DWI sequences to use, particularly to optimize the microstructural characterization of lesions. We evaluated the effects of demyelination and axonal loss on DKI and NODDI metrics and the impact of the sequence on the sensitivity to damage by performing a Monte-Carlo diffusion simulation inside a novel model of damaged WM. The sequence strongly affected the means and sensitivities of the metrics. Consequently, comparing DKI and NODDI analysis employing different sequences could lead to erroneous conclusions regarding the damage assessment: further investigations are needed for a community consensus on acquisition details.
Introduction
There is a general lack of agreed-upon guidelines regarding the sequence to use for each specific DWI multi-shell technique, in particular, to optimize the microstructural characterization of lesioned tissues: not many studies have proposed optimal experimental designs for multi-shell techniques and all of them only considered healthy tissue [1-5]. The purpose of this simulation study is to explore, for the first time, the impact of the acquisition protocol on the ability of two clinically feasible DWI multi-shell techniques (DKI and NODDI) to reveal demyelination and axonal loss, two damage processes typical of many neurological diseases [6-9].Methods
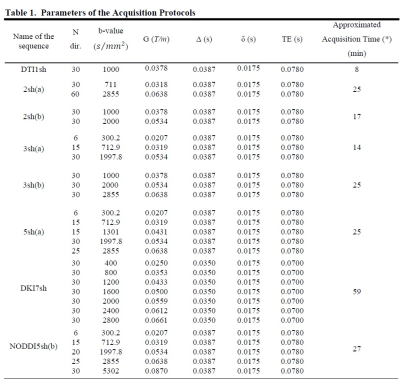
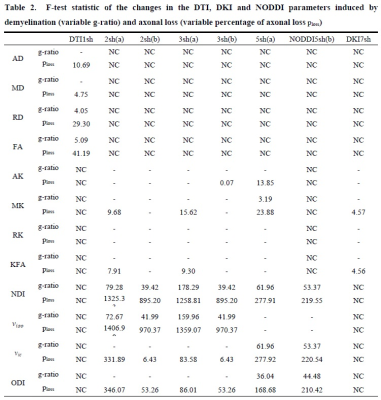
We developed a new synthetic WM model with parallel myelinated axons, including for the first time permeable membranes and axonal debris, with gamma-distributed radii in the presence of demyelination and axonal loss. We modeled demyelination by leaving unchanged the distribution of the axonal inner radii and increasing the g-ratio, as well as, accordingly, the myelin transmission probability, considering, that the probability of water molecules crossing the myelin sheath depends on thickness. We modeled axonal loss by reducing the axonal density, while selectively eliminating axons with smaller radii, considering the experimental findings reported in post-mortem and in-vivo studies on MS lesions [10-15], and considering, that extra-axonal diffusivity is affected by axonal debris occurring together with axonal loss. Thus, we performed a Monte Carlo simulation of water diffusion motion inside 21 synthetic voxels of WM with the above features (called substrates), and with different degrees of damage; we, then, calculated the DW signal from each substrate with various acquisition protocols (Table 1). For each DWI metric, damage process, and protocol, we performed one-way analysis of variance (ANOVA) and post hoc Tukey’s test to assess the effects on the metrics of different damage degrees. Significant metric changes between the damaged and healthy conditions were accepted at p < 0.01. We used the F statistic as an index of sensitivity to summarize the results of the statistical analysis since its greater values reflect better results of the post hoc Tukey test and, consequently, greater sensitivity.Results and Discussion
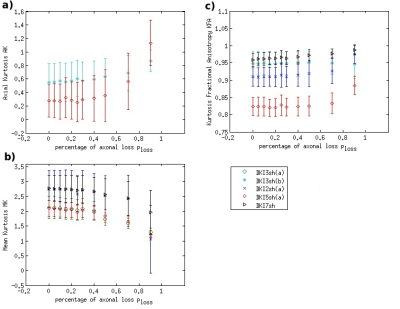
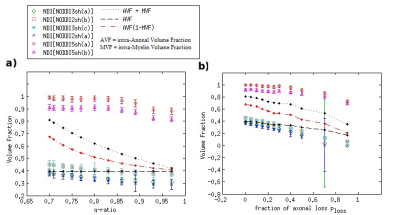
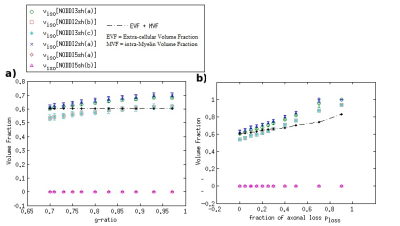
DKI metrics significantly changed between the healthy and damaged conditions in the presence of axonal loss, while no significant variation was observed due to demyelination, except for the mean kurtosis MK when using the acquisition protocol DKI5sh(a). These findings are consistent with those of many studies on MS patients [16-19]. However, the acquisition protocol impacted the metric averages (Figure 1c) and, especially, on the metric sensitivity to damage in an unexpected way (Table 2): 1) a DKI metric can be sensitive to the axonal loss with a given protocol but not with another; 2) the best sensitivities were obtained (with MK) when using a five-, rather than a seven-shell sequence. Such dependence on the protocol of the metric sensitivity to the damage could stem from the influence of both b-values and tissue type on the DKI metrics [2, 20]. In detail, sensitivity to damage depends on the variation of a parameter between different conditions of damage, namely in tissues with different microstructural features, that affect the parameters in different ways, depending on the b-value. Regarding NODDI, all the metrics showed high sensitivity to demyelination and excellent sensitivity to axonal loss. In this case, also, the acquisition protocol strongly impacted the metric means and sensitivity to damage. The largest differences in terms of both sensitivity and mean values occur between the results obtained with five-shell sequences and those obtained with two- and three-shell sequences (see Table 2 and Figures 2 and 3): the best sensitivities are achieved by the fractional volumes NDI (neurite density index) and νiso (isotropic volume fraction) with 2sh(a) and 3sh(a). Notably, our simulations allow comparing the average values of the NODDI metrics, with some microstructural features of the synthetic substrate. It appears that it is not feasible to estimate, at the same time, all the true intra-cellular, extra-cellular, and CSF fractional volumes with good accuracy with NODDI due to its simplified model of the microstructure lacking the myelin compartment. Up to three shells, NODDI models the signal coming from the intra-axonal space of the substrate as coming from the intracellular space. It models the rest of the signal mainly as coming from CSF, failing to identify in the substrate the extra-cellular space. In the case of five shells, the greater complexity of the data allows NODDI to rule out CSF (which is not present in the substrate), but then the signal is modeled as coming mostly from the intracellular compartment.Conclusion
We found that the acquisition protocol has a strong influence on DKI and NODDI metrics and particularly on their sensitivity to damage. Consequently, comparing experimental results of such diffusion analyses relying on different acquisition sequences could lead to erroneous conclusions regarding the presence or severity of the damage. As an alternative and more pragmatic clinical approach DKI and NODDI metrics could be viewed as biomarkers without considering the relationship to their ground truth values: further investigations are needed to find a community consensus on acquisition details to optimize the sensitivity to a given pathology.Acknowledgements
No acknowledgement found.References
1- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61:1000–1016.
2- Chuhutin A, Hansen B, Jespersen SN (2017) Precision and accuracy of diffusion kurtosis estimation and the influence of b-value selection. NMR Biomed 30(11).
3- Hutchinson EB, Avram AV, Irfanoglu MO, Koay CG, Barnett AS, Komlosh ME, Ozarslan E, Schwerin SC, Juliano SL, C Pierpaoli (2018) Analysis of the Effects of Noise, DWI Sampling, and Value of Assumed Parameters in Diffusion MRI Models. Magn Reson Med 78:1767–1780.
4- De Santis S, Assaf Y, Evans CJ, Jones DK (2014) Improved precision in CHARMED assessment of white matter through sampling scheme optimization and model parsimony testing. Magn Reson Med 71:661–671.
5- Li CX, Patel S, Zhang X (2020) Evaluation of multi-shell diffusion MRI acquisition strategy on quantitative analysis using multi-compartment models. Quant Imaging Med Surg 10(4):824‐834.
6- Love S (2006) Demyelinating diseases. J Clin Pathol 59(11):1151‐1159.
7- Ellwardt E, Zipp F (2014) Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp Neurol 262:8–17.
8- Weinshenker BG (1996) Epidemiology of multiple sclerosis. Neurol Clin 14:291–308.
9- Dendrou, CA, Fugger L, Friese MA (2015) Immunopathology of multiple sclerosis. Nat Rev Immunol 15:545–558.
10- Lassmann H (2010) Axonal and neuronal pathology in multiple sclerosis: What have we learnt from animal models. Exp Neurol 225:2–8.
11- Ganter P, Prince C, Esiri MM (1999) Spinal cord axonal loss in multiple sclerosis: a post-mortem study. Neuropathol Appl Neurobiol 25:459–467.
12- Kutzelnigg A, Lassmann H (2014) Pathology of multiple sclerosis and related inflammatory demyelinating diseases. In: Douglas J. Goodin, editors. Multiple Sclerosis and related disorders. Elsevier, vol 122 p 32.
13- Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W (2000) Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 123:1174–83.
14- Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM (2001) Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiplesclerosis. Brain 124:1813–1820.
15- De Luca GC, Ebers GC, Esiri MM (2004) Axonal loss in multiple sclerosis: a pathological survey of the corticospinal and sensory tracts. Brain 127:1009-101
16- By S, Xua J, Boxb BA, Bagnatoc FR, Smith SA (2017) Application and evaluation of NODDI in the cervical spinal cord of multiple sclerosis patients. NeuroImage Clin 15:333–342.
17- Yoshida M, Hori M, Yokoyama K, et al (2013) Diffusional kurtosis imaging of normal-appearing white matter in multiple sclerosis: preliminary clinical experience. Jpn J Radiol 31:50–55.
18- Raz E, Bester M, Sigmund EE et al (2013) A better characterization of spinal cord damage in multiple sclerosis: a diffusional kurtosis imaging study. Am J Neuroradiol 34:1846–1852.
19- Guglielmetti C, Veraart J, Roelant E, et al (2016) Diffusion kurtosis imaging probes cortical alterations and white matter pathology following cuprizone induced demyelination and spontaneous remyelination. NeuroImage 125:363–377.
20- Hutchinson EB, Avram AV, Irfanoglu MO, Koay CG, Barnett AS, Komlosh ME, Ozarslan E, Schwerin SC, Juliano SL, C Pierpaoli (2018) Analysis of the Effects of Noise, DWI Sampling, and Value of Assumed Parameters in Diffusion MRI Models. Magn Reson Med 78:1767–1780.
Figures