2810
Paramagnetic Rim Lesions in MS are characterized by heterogeneous damage and inflammatory activity: a combined T1 relaxometry-diffusion study1Translational Imaging in Neurology (ThINk) Basel, University Hospital Basel and University of Basel, Basel, Switzerland, 2Neurologic Clinic and Policlinic, University Hospital Basel and University of Basel, Basel, Switzerland, 3Research Center for Clinical Neuroimmunology and Neuroscience (RC2NB) Basel, University Hospital Basel and University of Basel, Basel, Switzerland, 4Institute of Neuropathology, University Medical Center, Göttingen, Germany, 5Department of Computer Science, University of Verona, Verona, Italy, 6Translational Neuroradiology Section, National Institute of Neurological Disorders and Stroke, Bethesda, MD, United States, 7Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 8Department of Neurology, Lausanne University Hospital, Lausanne, Switzerland, 9Cliniques universitaires Saint Luc, Université catholique de Louvain, Louvain, Belgium, 10F. Hoffmann-La Roche Ltd., Basel, Switzerland, 11Pharmaceutical Research and Early Development, Roche Innovation Center Basel F. Hoffmann-La Roche Ltd., Basel, Switzerland
Synopsis
Paramagnetic rim lesions (PRL) are characterized by heterogeneous damage in multiple sclerosis (MS) patients. In this study we acquired in vivo, postmortem and histopathology with the aim to further characterize the microstructural properties of these lesions. Furthermore, we disentangled the heterogeneity of PRL by combining a stick-ball-sphere diffusion model with the quantification of mean T1 relaxation times.
Introduction
Lesions presenting a paramagnetic rim on susceptibility-sensitive MRI (paramagnetic rim lesions-PRL) are characterized by chronic inflammatory activity1 and are associated with increased disability (Absinta et al. 2019) and neuroaxonal damage2 in patients with Multiple Sclerosis (MS). Histopathologically, PRL features a rim of activated microglia/macrophages at the edge, and various degrees of inflammatory demyelinating activity and infiltration of activated microglia/macrophages in the lesion core. In this work, we aimed at disentangling some characteristics of PRL by combining a measure of extra-cellular restricted fractions derived from multi-shell diffusion data with the quantification of mean T1 relaxation times from MP2RAGE. The diffusion model was chosen based on histopathological characteristics of PRL lesions, which feature pronounced axonal damage (and hence a strong loss of anisotropy) in the lesion core.METHODS
We acquired MRI images of 102 MS patients on a 3T MRI. Among those, 46 patients presented PRL and are included in this work: 37 Relapsing-remitting, 5 Secondary Progressive, 4 Primary Progressive; mean age 42±13; female 27; disease duration 7.82±7.92 years; median Expanded Disability Status Scale 2.5.Diffusion-weighted MRI (DW-MRI) data were acquired with resolution 1.8mm isotropic and the following b-values [0,700,1000,2000,3000] s/mm2 and number of measurements [12,6,20,45,66], repetition time (TR)=4.5s, echo-time (TE)=75ms, δ=19.2ms, Δ=36.5ms. MP2RAGE images4 with 1mm isotropic resolution, TR=5s, inversion times TI1=0.7s and TI2=2.5s, TE=2.98ms, provided T1 relaxometry maps. 3DFLAIR with isotropic resolution 1mm, TR=5s, TE=386ms, TI=1.8s was used for lesion segmentation. An additional 3DEPI, with isotropic resolution 670μm, TR=64ms, TE=35ms, was acquired to assess for the presence of PRL, and a Quantitative Susceptibility Mapping (QSM) reconstruction of the same EPI data was performed5. In one SPMS postmortem patient, an ex vivo dedicated 3D EPI6 with isotropic resolution 330μm, TR=65ms, TE=35ms and a dedicated MP2RAGE with isotropic resolution 670μm, TR=5s, TE=1.78ms, TI1=194ms and TI2=2.5s were also acquired. Two rim lesions identified with QSM applied to postmortem 3DEPI were then stained using Myelin Basic Protein for myelin and CR3/43 for microglia/macrophages. DWI-MRI data were processed with denoising, eddy currents, susceptibility and motion. White matter (WM) lesions were segmented using a CNN method7 and manually corrected by an expert neuroradiologist. Two experts identified 226 PRL. A dictionary-based DW-MRI model was applied to characterize the tissue components of lesions. The method was implemented in the AMICO framework8. A three-compartment model with the following parameters for the dictionary was implemented: stick with diffusivity 2.0μm2/ms, ball with diffusivities [2.0,3.0]μm2/ms, and spheres with radius [3.0,10.0]μm and 11 atoms with diffusivities, one atom with [0.01]μm2/ms and 10 equally-spaced atoms in the range [0.25,2.5]μm2/ms.RESULTS
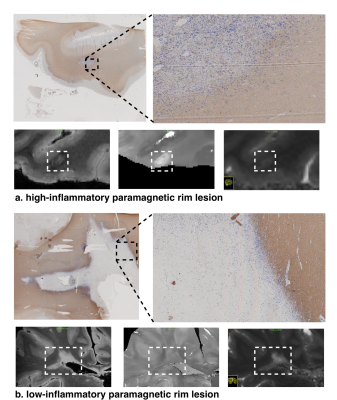
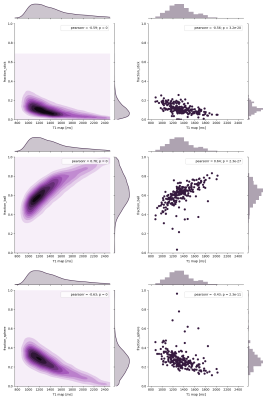
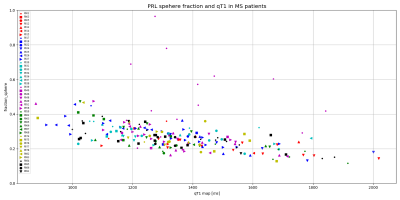
In Figure1, we show a histological sample of two very different PRL stained with MBP and CR3/43 (MHC II): Figure1a shows a PRL with activated microglia/macrophages both at the edge and in the center of the lesion (purple) and figure 1b shows a rim of activated microglia/macrophages but scarce cellularity in the center. In the bottom of Figure1a and Figure1b, we show a patch of a 3DEPI, QSM and a qT1 images of the exemplary highly-inflammatory PRL (a) and low-inflammatory PRL (b). Those have respective qT1 values of 355ms±36 vs 535ms±116 (mean±SD). In Figure2, we performed a voxel-wise, and lesion-wise analysis of the DWI-based stick-ball-sphere fractions and qT1 in 226 PRL identified in vivo in MS patients. The voxel-wise analysis, which included all PRL voxels, showed a Pearson correlation coefficient of -0.63 between sphere fraction and qT1. qT1 in PRL in vivo is much longer than under ex vivo conditions due to the shortening of qT1 after fixation in the postmortem experimental setting. The lesion-wise correlation, which was performed using the mean value of sphere fraction and qT1 in PRL, showed a Pearson correlation coefficient of -0.43. In Figure3, we report the mean values of qT1 and sphere fraction using a different shape and colour for each patient, in order to show the heterogeneity of the PRL characteristics in single patients with MS.DISCUSSION
Using measures of both qT1 and sphere-fraction derived from diffusion MRI, we provide evidence that PRL structurally heterogeneous in vivo in MS patients. Our data show that- in PRL -a wide range of qT1 corresponds to different levels of sphere fractions, as measured with a three-compartment model. Short qT1 in PRL corresponded to higher values of sphere fractions, which might correspond to a high concentration of activated microglia/macrophages of highly inflammatory PRL (Figure 1a) or eventually to axonal spheroids due to ongoing axonal damage. On the other hand, PRL lesions with long qT1 values are associated to lower sphere-fractions and higher fractions of ball-fractions in PRL, suggesting an extensive tissue damage but also the scarce presence of inflammatory cells/axonal spheroids, as shown in the exemplary lesion represented in Figure 1b.CONCLUSION
In this work, we used histological, postmortem and in vivo MRI to characterize the heterogeneity of PRL. We proposed a diffusion model intending to disentangle microstructural characteristics of PRL, which -together with qT1- might help to identify PRL with more pronounced chronic inflammatory activity and with prevalent tissue loss and scarce inflammation. Future work will aim at exploring an extension of the applied diffusion model and include new DW-MRI acquisition-schemes, in order to gain further understanding in the stratification of MS lesions characteristics.Acknowledgements
No acknowledgement found.References
1 Dal-Bianco et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathologica. 2017
2 Absinta et al. Association of Chronic Active Multiple Sclerosis Lesions With Disability In Vivo. JAMA Neurol. 2019
3 Maggi et al. Paramagnetic phase rims and serum neurofilaments in relapsing-remitting and progressive multiple sclerosis patients: a combined laboratory-imaging marker of chronic inflammation. ECTRIMS 2019
4 Marques et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 2010
5 Liu et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage 2012
6 Weigel et al. Postmortem Whole-Brain MP2RAGE Optimization at 3T: A New Imaging Window into Multiple Sclerosis Cortical Pathology. Proceedings of the ISMRM 2020, p. 1759.
7 La Rosa et al. Multiple sclerosis cortical and WM lesion segmentation at 3T MRI: a deep learning method based on FLAIR and MP2RAGE. NeuroImage: Clinical 2020
8 Daducci et al. Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. NeuroImage 2015
Figures