2809
Relevance analysis of identifying multiple sclerosis patients based on diffusion imaging data using CNN1Medical Physics Group / IDIR, Jena University Hospital, Jena, Germany, 2Michael-Stifel-Center for Data-Driven and Simulation Science, Jena, Germany, 3Department of Neurology, Medical University of Graz, Graz, Austria, 4Center of Medical Optics and Photonics Jena, Jena, Germany
Synopsis
To analyze the classification procedure of identifying multiple sclerosis (MS) based on diffusion-weighted imaging data by using convolutional neural networks (CNNs), we generated relevance maps. The relevance maps indicate the contribution of each input voxel to the final classification score and may facilitate new findings regarding MS-specific biomarkers. The study showed that voxels in the central brain area including some of the lesion voxels are important for correct classification. This information may be used in the future to perform a more detailed analysis in order to classify different MS-phenotypes or predict disease progression.
Introduction
Diffusion magnetic resonance imaging (dMRI) allows examining microstructural changes occurring in multiple sclerosis (MS) that are more specific to the disease than conventional T1- or T2-weighted imaging1. The intracellular volume fraction (ICVF), being one of the advanced dMRI measures, refers to the axon and dendrite density and is used to evaluate the distribution of white matter lesions in MS patients in comparison to healthy controls (HC)2. Here, we aimed to classify MS patients and HC based on ICVF data using a convolutional neural network (CNN). Subsequently, we analyzed the relevant voxel information that contributed to the classification decisions using the DeepLIFT algorithm3. This relevance analysis4 helps to discover new MS markers or to confirm the current ones, thus supporting CNN-based clinical applications.Materials and Methods
Diffusion MR imaging was performed with 64 MS patients and 64 HC using a 3T MRI (Siemens Prisma, 20 channel head coil). The MR diffusion acquisition used a multi-shell and multi-slice (SMS-slice-factor= 4) protocol with 1.5 mm isotropic resolution. The diffusion series was measured twice with reversed phase-encoding polarity to facilitate compensation of susceptibility induced geometric deformation. The microscopic diffusion anisotropy parameter ICVF was computed using the spherical mean technique (SMT5). For all subjects, we selected a two-dimensional image in transverse orientation at a predefined slice position from the three-dimensional ICVF volume. The resulting dataset of ICVF images was randomly split into a training group with 32 MS and 32 HC samples and a testing group with the remaining 64 samples.To classify patients and controls, we used the following network architecture4. The 2D CNN had five convolutional layers (filter sizes = 16, 16, 32, 32, 64; kernel size = 3×3) and two fully connected layers (number of neurons = 8, 2) with rectified linear unit (ReLU) activation functions. For the training procedure, a data augmentation generator was used to perform image rotation, horizontal and vertical shifting, scaling, and horizontal flipping to a batch of samples. The trained CNN model showed sufficient performance on the test set (80% accuracy) and was used for further analysis.
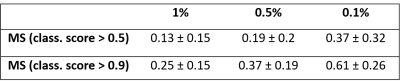
After network model training, we applied the DeepLIFT algorithm3 to assign the relevance to the input image voxels in their ability to explain the contribution of these voxels to the output classification score. These relevance maps were qualitatively evaluated on a single subject and a population-based basis. To analyze the significance of lesion information for the classification, we extracted FLAIR-based lesion maps, which were initially defined using LST6 and manually adjusted by expert readers. We compared these lesion maps with the relevance maps for all correctly predicted MS patients and only for patients with the highest classification score (>0.9). We used the mean intersection over union (IoU) score as an evaluation metric in order to evaluate how much of the FLAIR-based lesion voxel contribute to the ICVF-based classification.
Results
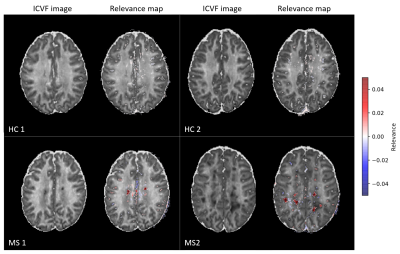
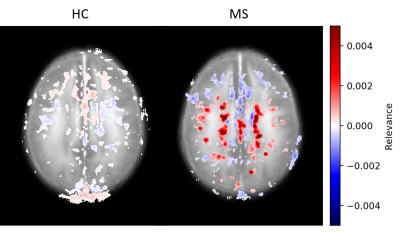
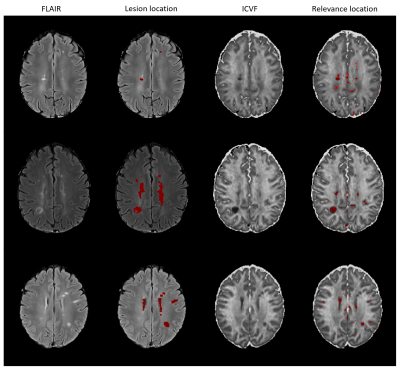
Figure 1 shows the relevance maps thresholded for the first and the last percentile for two correctly predicted HC and two correctly predicted MS. We see that the relevance values for HC are smaller than for MS. For MS subjects, positive relevance is distinctly more pronounced and located in the central brain region. The higher degree of relevance for the MS population as well as the location of the most relevant voxels is well seen on the average maps in Figure 2.FLAIR based lesion maps and corresponding ICVF-based positive relevance maps are shown for three MS patients in Figure 3 (in rows). We see that most of the relevant areas correspond to the lesions in all three subjects. Table 1 summarizes the mean IoU score across the subjects. The IoU increases when increasing the positive relevance threshold and is higher when computed only for MS patients with high classification accuracy.
Discussion and Conclusion
In our study, we identified MS patients using CNN on ICVF data. The CNN decision strategy was analyzed using DeepLIFT relevance maps. The relevance maps revealed that voxels in the central brain area contribute mostly to the classification. By comparing the lesion maps and the relevance maps of MS subjects, we find that the lesion voxel information in ICVF images is obviously important for the CNN classification. Specifically, we observed a relation between the high classification score and the high relevance of the voxels matching with the lesion voxels in MS patients. For future studies, the discrepancy between voxel locations identified as FLAIR-based lesion and relevant voxels for ICVF-based classification should be analyzed.The study has some limitations. First, for the current study the performance of the model appears sufficient, but for future studies, we suggest to hyper-tune the parameters of the CNN and generate more specific relevance maps. A second limitation is the use of lesion maps derived from FLAIR images. Since the lesion information can differ between the two contrasts (FLAIR and ICVF), future combination of FLAIR-based and ICVF-based lesion maps might enable a more robust analysis.
Acknowledgements
This study was supported in parts by the Carl-Zeiss-Foundation (CZ-Project: Virtual Workshop), the German Research Foundation (RE1123/21-1), and the Austrian Science Fund (FWF3001-B27).References
1. Mustafi S, Harezlak J, Kodiweera C, et al. Detecting white matter alterations in multiple sclerosis using advanced diffusion magnetic resonance imaging. Neural Regen Res. 2019;14(1):114-123. doi:10.4103/1673-5374.243716
2. Hagiwara A, Kamagata K, Shimoji K, et al. White matter abnormalities in multiple sclerosis evaluated by quantitative synthetic MRI, diffusion tensor imaging, and neurite orientation dispersion and density imaging. Am J Neuroradiol. 2019;40(10):1642-1648. doi:10.3174/ajnr.A6209
3. Shrikumar A, Greenside P, Kundaje A. Learning Important Features Through Propagating Activation Differences. Proceedings of the 34th International Conference on Machine Learning. Sydney; 2017:3145–3153.
4. Lopatina A, Ropele S, Sibgatulin R, Reichenbach JR, Güllmar D. Investigation of Deep-Learning-Driven Identification of Multiple Sclerosis Patients Based on Susceptibility-Weighted Images Using Relevance Analysis. Front Neurosci. 2020;14:1-12. doi:10.3389/fnins.2020.609468
5. Kaden E, Kruggel F, Alexander DC. Quantitative mapping of the per-axon diffusion coefficients in brain white matter. Magn Reson Med. 2016;75(4):1752-1763. doi:10.1002/mrm.25734
6. Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59(4):3774-3783. doi:10.1016/j.neuroimage.2011.11.032
Figures