2808
Prediction of multiple sclerosis clinical progression using whole brain adiabatic T1rho and Relaxation Along a Fictitious Field imaging1Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Turku University Hospital, Turku, Finland, 3University of Turku, Turku, Finland, 4University of Oulu, Oulu, Finland
Synopsis
In this single center prospective clinical trial, we have shown feasibility of whole brain of T1ρadiab and TRAFF2 at 3T. Both of these methods provided higher lesion-to-normal appearing white matter contrast compared with conventional used T1-weighted imaging, and demonstrated potential to predict multiple sclerosis disease severity scores (EDSS, MSSS) at the time of imaging and 1-year follow-up. These encouraging findings stimulate further application of T1ρadiab and TRAFF2 at 3T in patients with multiple sclerosis.
INTRODUCTION
Relaxation along a fictitious field (RAFF) is an MRI technique applying amplitude and frequency-modulated irradiation in a subadiabatic regime (1–4). Rotating frame relaxations (T1ρ and TRAFF) have shown to be quantitative MRI markers for brain myelin content (5), to follow up disease progression, including brain and myocardial ischemia (6), and to follow up response to therapy (7). However. there is only limited data on applicability of these methods using clinical high field MR scanners (8). In the current study, we aimed to explore i) feasibility of rotating frame imaging (T1ρ and TRAFF) in multiple sclerosis (MS) patients using a clinical 3 Tesla (T) MR scanner, ii) explore differences in relaxation values of different tissues in MS patients, and iii) explore correlation of relaxation values with MS disease severity.METHODS
General physical examination and detailed neurological examination (The Expanded Disability Status Scale, EDSS; The Multiple Sclerosis Severity Score, MSSS) was performed at the time of MRI scan and one year after completion of the trial.The MRI examinations were performed using 3T Philips system (Philips Ingenia, Best, Netherlands). Both T1ρ and TRAFF were measured using 3D T1-FFE sequence with the following parameters: TR/TE 4.1/2.3 ms, FOV 240x240x141 mm3, acquisition voxel size 1.5x1.5x3.0 mm3, reconstruction voxel size of 1.5x1.5x1.5 mm3, TFE factor 20, centric k-space coding. Adiabatic T1ρ (T1ρadiab) was acquired using hyperbolic secant pulses with radiofrequency (RF) peak amplitude 575 Hz (corresponding to 13.50 mT B1) and pulse duration 12 ms. The pulse train durations were 72 ms and 144 ms. Second order RAFF (RAFF2) was performed with RF peak amplitude of 500 Hz (corresponding to 11.74 mT B1) and pulse train durations 68 ms and 136 ms. In addition, 3D T2-weighted, FLAIR and T1- weighted images were acquired.
The relaxation time values of adiabatic T1r (T1ρadiab), RAFF2 (TRAFF2) were calculated using two parameter monoexponential model. Image post-processing was performed using FSL (9) 5.0.4 with SPM8 and VBM8 toolbox used in co-registration, re-slicing and segmentation of T1-weighted, T2-weighted and FLAIR images. T1-weighted images were segmented to GM, WM, Cerebrospinal Fluid using VBM8 toolbox and subcortical regions were excluded from GM and WM masks using FIRST (10) tool segmentations. LST Toolbox (version 1.2.3) (11) was used to segment lesions using T1 and FLAIR images.
Correlation of T1ρadiab and TRAFF2 relaxation values to EDSS and MSSS) at baseline and 1-year after the scan, were evaluated using regression analysis, correcting for an effect of age. Correspondingly, change in EDSS/MSSS within 1-year was evaluated using a logistic regression model. P-values below 0.05 after correction for multiple comparisons were considered statistically significant, unless otherwise noted. All statistical tests were done in RStudio environment (v 1.1.383. 2017 RStudio Inc.).
RESULTS
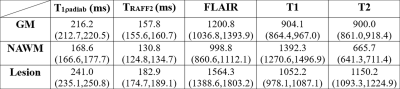
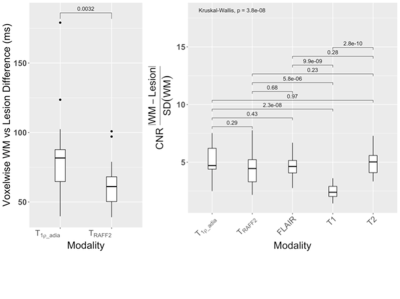
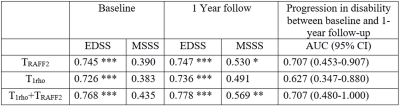
In total, 21 patients were included in the final analyses. Statistically significant differences in TRAFF2 values were present between the tissue types in MS patients with median (interquartile range) over patients TRAFF2 values for NAWM, GM, and lesions 131 (125-135), 158 (156-161), and 183 (175-189) ms (Figure 1). Similarly, statistically significant differences in T1ρadiab values were present between the tissue types in MS patients with median (interquartile range) T1ρadiab values for NAWM, GM, and lesions of 216 (167-178), 169 (213-221), and 241 (235-251) ms (Figure 1). T1ρadiab was found to have the second highest median lesion-to-normal appearing white matter ratio (4.7) after T2 (5.0) (Figure 2).Statistically significant correlation was found between the TRAFF2 median relaxation of MS lesion value and EDSS, MSSM at the baseline as well at the 1-year follow-up (Figure 3), while these correlations were significant for only EDSS with reasonably high coefficient of determinations in 0.73 to 0.78. TRAFF2 and T1ρadiab, individually or in combination, demonstrated potential for prediction of disease progression in EDSS at 1-year follow-up with AUC (95% CI) of 0.71 (0.45-0.91) and 0.63 (0.35-0.88), Figure 3, respectively. When evaluating model including both TRAFF2 and T1ρadiab, T1ρadiab was not found to have benefit over using TRAFF2 alone (p>0.160).
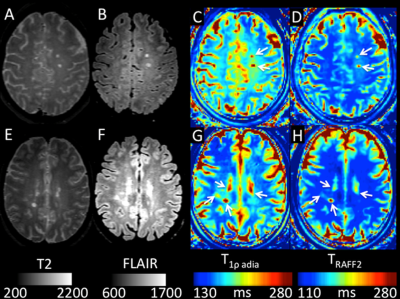
Representative imaging findings of MS patients are shown in Figure 4.
DISCUSION & CONCLUSION
T1ρadiab and TRAFF2 demonstrated potential to predict MS disease severity scores (EDSS, MSSS) at the time of imaging and 1-year follow-up, specifically TRAFF2 values were more predictive. These findings suggest that T1ρadiab and TRAFF2 could potentially serve as non-invasive imaging biomarkers of disease progression.Acknowledgements
The authors wish to acknowledge CSC – IT Center for Science, Finland, for computational resources used in creation of the parameter maps in this study. This study was financially supported by grants from Instrumentarium Research Foundation, Sigrid Jusélius Foundation, Turku University Hospital, TYKS-SAPA research fund, Finnish Cultural Foundation, and Orion Research Foundation. HM was supported by the Cultural Foundation of Finland, and Orion Pharma Research Fellowship.References
1. Liimatainen T, Mangia S, Ling W, et al. Relaxation dispersion in MRI induced by fictitious magnetic fields. J Magn Reson. Elsevier Inc.; 2011;209(2):269–276http://dx.doi.org/10.1016/j.jmr.2011.01.022.
2. Liimatainen T, Sorce DJ, O’Connell R, Garwood M, Michaeli S. MRI contrast from relaxation along a fictitious field (RAFF). Magn Reson Med. 2010;64(4):983–994.
3. Liimatainen T, Sierra A, Hakkarainen H, et al. MRI Contrasts Generated Using Fictitious Fields in High-Rank Rotating Frames Correlate With Myelin Content in Normal Rat Brain ex vivo. Proc 21st Sci Meet Int Soc Magn Reson Med. 2013;21:0869.
4. Liimatainen T, Hakkarainen H, Mangia S, et al. MRI contrasts in high rank rotating frames. Magn Reson Med. 2015;73(1):254–262.
5. Hakkarainen H, Sierra A, Mangia S, et al. MRI relaxation in the presence of fictitious fields correlates with myelin content in normal rat brain. Magn Reson Med. 2016;75(1):161–168.
6. Grohn OH, Lukkarinen JA, Silvennoinen MJ, Pitkanen A, van Zijl PC, Kauppinen RA. Quantitative magnetic resonance imaging assessment of cerebral ischemia in rat using on-resonance T(1) in the rotating frame. Magn Reson. p. 268–276.
7. Hakumäki JM, Gröhn OHJ, Tyynelä K, Valonen P, Ylä-Herttuala S, Kauppinen RA. Early gene therapy-induced apoptotic response in BT4C gliomas by magnetic resonance relaxation contrast T1 in the rotating frame. Cancer Gene Ther. 2002;9(4):338–345.
8. Filip P, Svatkova A, Carpenter AF, et al. Rotating frame MRI relaxations as markers of diffuse white matter abnormalities in multiple sclerosis. NeuroImage Clin. Elsevier Inc.; 2020;26https://pubmed.ncbi.nlm.nih.gov/32272373/. Accessed November 30, 2020.
9. Hernández M, Guerrero GD, Cecilia JM, et al. Accelerating Fibre Orientation Estimation from Diffusion Weighted Magnetic Resonance Imaging Using GPUs. PLoS One. 2013;8(4):61892.
10. Amann M, Andělová M, Pfister A, et al. Subcortical brain segmentation of two dimensional T1-weighted data sets with FMRIB’s Integrated Registration and Segmentation Tool (FIRST). NeuroImage Clin. Elsevier Inc.; 2015;7:43–52.
11. Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. Academic Press; 2012;59(4):3774–3783.
Figures