2804
Automatic Segmentation of Diffusely Abnormal White Matter in MS Using Deep Neural Network1Diagnostic and Interventional Imaging, University of Texas Health Science Center at Houston, Houston, TX, United States
Synopsis
Deep neural network was used to automatically segment diffusely abnormal white matter (DAWM) in 100 relapsing remitting multiple sclerosis patients (RRMS). Our calculated DAWM prevalence of 32% is comparable to ~ 25% reported elsewhere. Based on our studies, only 13% of T2 lesions at baseline converted into DAWM by 60 months. Of the DAWM detected at baseline, only 15% converted to lesions, 45% persisted, and 40% resolved (converted to NAWM). These initial results suggest that DAWM may present a significant disease burden by itself.
Introduction
In addition to focal hyperintense lesions on T2-weighted images, diffusely abnormal white matter (DAWM) that appears as moderately hyperintense regions with poorly defined margins is frequently seen on MRI in multiple sclerosis (MS) patients.1 The signal intensity of DAWM is close to that of gray matter (GM) and in-between the intensities of focal lesions and normal appearing white matter (NAWM).2 Prior studies suggest that DAWM is present early on in the disease.3 Histopathology observations suggest that DAWM exhibits axonopathy, demyelination, and fibrillary gliosis3, 4, with significantly different characteristics from focal lesions, may be a significant contributor to disease progression, and could serve as a biomarker of neurodegeneration.5 These studies strongly suggest an important role of DAWM in MS pathology and clinical disability. Despite its potentially important role in MS pathology, DAWM is notoriously difficult to segment on MRI due to absence of easily defined cut-off that separates its signal intensity from surrounding tissues.6 Lack of reliable segmentation has significantly slowed progress in characterizing and understanding the role of DAWM in MS. The purpose of this study is to apply deep convolutional neural networks7 to segment DAWM in relapsing remitting multiple sclerosis (RRMS) patients.Methods
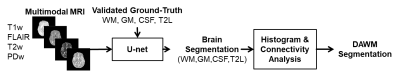
We analyzed the MRI data acquired as a part of CombiRx, a phase III, multi-center trial for evaluating combination treatment in 1008 RRMS patients8 and MRIs a were acquired at multiple time points on multiple MRI platforms.9 The imaging protocol included 3D T1w images, 2D FLAIR, dual-echo fast spin echo, and pre- and post-contrast T1w images.The workflow for segmentation of DAWM is shown in Fig. 1. Initially the U-net (Fig. 1) was trained to segment brain into WM, GM, CSF, and T2 lesions using FLAIR, duel echo, pre-contrast T1-weighted images as the input.10 A two-step procedure was implemented to segment DAWM in 100 randomly chosen scans. For each voxel, the soft-max activation function at the output layer of the U-net assigns scores between 0 and 1 for each tissue class, representing the likelihood that a voxel belongs to the class. The log-transformed scores of the T2 lesions class of all voxels initially segmented as WM or T2 lesions were examined for possible DAWM assignment. The criteria for DAWM assignment was (i) WM voxels with T2 lesion (T2L) scores above the 50-percentile of the scores of GM or (ii) T2 lesions voxels with T2L scores below 95-percentile of that of WM. Connectivity analysis was performed to remove false positives. The initial WM and T2 lesion masks were subsequently updated to remove DAWM voxels. The prevalence of DAWM was computed as the percentage of subjects with non-zero DAWM volume at baseline. For determining the interconversion frequency between lesions and DAWM, images up to the 60-month visit were first aligned to the baseline scan before processing.
Results
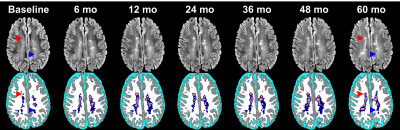
Segmentation results from one MS patient are shown in Fig. 2. As can be seen from Fig. 2, the two-step procedure has done a credible job in segmenting DAWM as judged visually by two neuroimaging experts. In this subset, we observed that DAWM is frequently present adjacent and prior to formation of focal lesions, but is also present without lesions as shown in Fig.2. We observed a prevalence of 32% for DAWM in these 100 patients. Only 13% of T2 lesions at baseline converted into DAWM by the last visit. Of the DAWM detected at baseline, only 15% converted to lesions, 45% persisted, and 40% resolved (converted to NAWM).Discussion
An automatic method based on deep neural network was implemented to segment DAWM on MRI acquired at multiple centers on different MRI platforms operating at different strengths. With the exception of two recent studies3, 11, majority of the publications on DAWM in MS were either from a single center or on a small number of patients. Our results are expected to be more robust with greater generalizability than a single center study with a small sample size. Our calculated DAWM prevalence of 32% is comparable to ~ 25% reported elsewhere.3, 4 These initial results suggest that DAWM may present a significant disease burden by itself.Conclusions
We have implemented a fully automatic segmentation technique based on deep neural network for quantifying DAWM and its prevalence and tissue interconversion. The segmentation results look highly promising based on visual inspection by experts. The ability to automatically segment DAWM using conventional MRI sequences should be of considerable help in patient management and conducting clinical trial.Acknowledgements
We thank John Lincoln and Arash Kamali for valuable discussions.References
1. Zhao, G. et al. Possible prognostic significance of dirty-appearing white matter on MRI in multiple sclerosis. Mult Scler. 2003; 9:S61.
2. Moore, G. R. W. et al. Dirty-appearing white matter in multiple sclerosis: Preliminary observations of myelin phospholipid and axonal loss. J. Neurol. 2008;255:1802
3. Vertinsky, A. T. et al. Diffusely Abnormal White Matter, T2 Burden of Disease, and Brain Volume in Relapsing-Remitting Multiple Sclerosis. J. Neuroimaging. 2019;29:151.
4. Laule, C. et al. Pathological basis of diffusely abnormal white matter: Insights from magnetic resonance imaging and histology. Mult Scler. 2011;17:144..
5. Seewann, A. et al. Diffusely abnormal white matter in chronic multiple sclerosis: Imaging and histopathologic analysis. Arch Neurol. 2009;66:601
6. Filippi, M. & Rocca, M. A. Dirty-appearing white matter: A disregarded entity in multiple sclerosis. Am. J. Neuroradiol. 2010;31:390.
7. Goodfellow, I., Bengio, Y., Courville, A. & Bengio, Y. Deep learning. vol. 1 (MIT press Cambridge, 2016).
8. Lindsey, J. W. et al. The CombiRx trial of combined therapy with interferon and glatiramer cetate in relapsing remitting MS: Design and baseline characteristics. Mult Scler Relat Disord. 2012; 1, 81).
9. Lublin, F. D. et al. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann Neurol. 2013;73:327.
10. Gabr RE, Coronado I, Robinson M, Sujit SJ, Datta S, Sun X, Allen WJ, Lublin FD, Wolinsky JS, Narayana PA. Brain and lesion segmentation in multiple sclerosis using fully convolutional neural networks: A large-scale study. Mult Scler. 2020;26(10):1217-26.
11. Dadar, M. et al.Conversion of diffusely abnormal white matter to focal lesions is linked to progression in secondary progressive multiple sclerosis. Mult. Scler. J. Mult Scle J. 2020 Mar 23;1352458520912172. doi: 10.1177/1352458520912172
Figures