2803
Assessing proximal and distal peripheral nerve damage in relapsing-remitting multiple sclerosis using magnetisation transfer ratio
Marios C. Yiannakas1, Ratthaporn Boonsuth1, Carmen Tur1,2, Marco Battiston1, Francesco Grussu1,3, Rebecca S. Samson1, Torben Schneider4, Masami Yoneyama5, Ferran Prados1,6,7, Sara Collorone1, Rosanna Cortese1, Olga Ciccarelli1, and Claudia A. M. Gandini Wheeler-Kingshott1,8,9
1NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Fuculty of Brain Sciences, University College London, London, United Kingdom, 2Multiple Sclerosis Centre of Catalonia (Cemcat), Vall d’Hebron Institute of Research, Vall d'Hebron Barcelona Hospital Campus, Barcelona, Spain, 3Radiomics Group, Vall d’Hebron Institute of Oncology, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain, 4Philips Healthcare, Surrey, Guildford, United Kingdom, 5Philips Japan, Minatoku, Tokyo, Japan, 6Centre for Medical Image Computing, Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 7Universitat Oberta de Catalunya, Barcelona, Spain, 8Department of Brain and Behavioural Sciences, University of Pavia, Pavia, Italy, 9Brain Connectivity Research Centre, IRCCS Mondino Foundation, Pavia, Italy
1NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, Fuculty of Brain Sciences, University College London, London, United Kingdom, 2Multiple Sclerosis Centre of Catalonia (Cemcat), Vall d’Hebron Institute of Research, Vall d'Hebron Barcelona Hospital Campus, Barcelona, Spain, 3Radiomics Group, Vall d’Hebron Institute of Oncology, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain, 4Philips Healthcare, Surrey, Guildford, United Kingdom, 5Philips Japan, Minatoku, Tokyo, Japan, 6Centre for Medical Image Computing, Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 7Universitat Oberta de Catalunya, Barcelona, Spain, 8Department of Brain and Behavioural Sciences, University of Pavia, Pavia, Italy, 9Brain Connectivity Research Centre, IRCCS Mondino Foundation, Pavia, Italy
Synopsis
Whilst multiple sclerosis (MS) is thought to be a disease of the central nervous system, evidence from neuropathological investigations has demonstrated that the peripheral nervous system (PNS) can also be affected in MS, with demyelination and axonal degeneration being the main pathophysiological mechanisms involved. In this study, PNS damage is assessed in vivo at proximal (lumbar plexus) and distal (sciatic nerve) anatomical locations in people with relapsing-remitting MS and healthy controls using magnetisation transfer ratio (MTR). Results demonstrate significantly reduced MTR values at distal anatomical locations, however no relationship is identified between these changes and clinical scores of disability.
INTRODUCTION
Peripheral nervous system (PNS) involvement in multiple sclerosis (MS) is an area that has received very little attention over the years, despite the evidence from neuropathological and biopsy reports confirming the presence of PNS damage at both proximal and distal anatomical locations in people with MS1-4. Given the discrepancies observed frequently between clinical symptoms and damage to the central nervous system (CNS), understanding the mechanisms of PNS damage and the interdependencies with CNS pathology could be invaluable in addressing this gap in knowledge. In this study, we investigated the lumbar plexus and the sciatic nerve of people with relapsing-remitting MS (RRMS) and healthy controls in vivo using magnetisation transfer ratio (MTR) to determine: a) the presence of PNS damage at proximal and distal anatomical locations and b) the relationship between MTR measures and clinical scores of disability.METHOD
1) Participants: Eleven healthy controls (HCs) (mean age 33.6 years, 7 female, range 24-50) and 15 people with relapsing remitting MS (RRMS) (mean age 38.5 years, 12 female, range 30-56) were recruited for this study. Informed consent was obtained from all participants and the study was approved by local ethics; 2) Clinical assessments: All people with RRMS were assessed using the expanded disability status scale (EDSS) by a qualified neurologist; 3) MRI protocol: A Philips Ingenia CX 3T was used with 28-channel anterior and posterior coils. For visualisation of the lumbar plexus and the sciatic nerve, the 3D SHINKEI sequence was used in the coronal plane5,6. For the lumbar plexus, MTR imaging was performed in the coronal plane using identical scan geometry to the 3D SHINKEI acquisition to obtain mean MTR values in the proximal L2-L5 segments. For the sciatic nerve, the upper 1/3 of the thigh was imaged using high-resolution fat-suppressed T2-weighted and MTR acquisitions in the axial plane; details of the MRI acquisition and analysis protocol were reported previously7; 4) Image analysis: Image segmentation was performed manually in FSLview (http://www.fmrib.ox.ac.uk/fsl/). For the lumbar plexus, each lumbar segment (L2-L5) was manually segmented on the 3D SHINKEI images, with separate binary masks created for the preganglionic, ganglionic and post ganglionic regions (Figure 1). The sciatic nerve was segmented manually on fat-suppressed T2-weighted images (Figure 2); 5) Statistical analysis: Statistical analysis was performed using STATA/SE 14.2 (StataCorp, College Station, TX). Linear regression models (adjusting for age and gender) were used to: a) identify differences in MTR values between HCs and people with RRMS at each anatomical location; b) identify relationships between MTR measures and clinical scores of disability (EDSS).RESULTS
In HCs, mean (±SD) MTR in all lumbar segments combined (L2-L5) was 28.0 (±5.6) and in people with RRMS was 28.8 (±5.2); this difference was not statistically significant (p=0.43). In addition, no statistically significant differences in MTR values were identified between the groups in the preganglionic, ganglionic and postganglionic regions (Figure 3). In the sciatic nerve, mean (±SD) MTR in HCs was 35.0 (±3.9) and in people with RRMS was 32.4 (±4.4); this difference was statistically significant (p=0.027) (Figure 4). Figure 5 shows the summary of all the MTR measurement in HCs and people with RRMS. The mean EDSS of the group was 1.7. No correlations were identified between the MTR measures and clinical scores of disability (EDSS) at any of the anatomical locations.DISCUSSION AND CONCLUSION
In this study we have demonstrated significant changes in MTR values in the sciatic nerve of people with RRMS as compared to HCs, but no changes were identified in the lumbar plexus. This finding demonstrates that pathological changes in myelin content can be present at distal locations of the PNS in people with RRMS, but maybe independent of changes at more proximal locations of the PNS. Furthermore, no relationship between the MTR measures in the PNS and clinical scores of disability (EDSS) were identified in this cohort of people with RRMS suggesting that more work is needed in the future to better understand the role of the PNS in explaining physical disability in MS.Acknowledgements
The UK MS Society and the UCL-UCLH Biomedical Research Centre for ongoing support. CGWK receives funding from the UK MS Society (#77), Wings for Life (#169111), Horizon2020 (CDS-QUAMRI, #634541), BRC (#BRC704/CAP/CGW). FP is a non-clinical Postdoctoral Guarantors of Brain fellow. This project has received funding under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 634541 and from the Engineering and Physical Sciences Research Council (EPSRC EP/R006032/1), funding FG. FG is currently supported by the investigator-initiated PREdICT study at the Vall d'Hebron Institute of Oncology (Barcelona), funded by AstraZeneca. CT receives the support of a fellowship from ”la Caixa” Foundation (ID 100010434). The fellowship code is LCF/BQ/PI20/11760008.References
- Hasson J, Terry R.D Zimmerman H.M. Peripheral neuropathy in multiple sclerosis. Neurology. 1958; 8(7):503-10.
- Miglietta O, Lowenthal M. A study of peripheral nerve involvement in fifty-four patients with multiple sclerosis. Arch Phys Med Rehabil. 1961;42:573-8.
- Pollock M, Calder C, Allpress S. Peripheral nerve abnormality in multiple sclerosis. Ann Neurol. 1977;2(1):41-8.
- Schoene W.C, Carpenter S, Behan P.O, et al. ‘Onion bulb’ formations in the central nervous system in association with multiple sclerosis and hypertrophic polyneuropathy. 1977;100(4):755-73.
- Yoneyama M, Takahara T, Kwee T.C, et al. Rapid high resolution MR neurography with a diffusion-weighted pre-pulse, Magn. Reson. Med. Sci. 2013;12(2):111–119.
- Kasper J.M, Wadhwa V, Scott K.M, et al. SHINKEI-a novel 3D isotropic MR neurography technique: technical advantages over 3DIRTSE-based imaging, Eur. Radiol. 2015;25(6):1672–1677.
- Yiannakas MC, Battiston M, Grussu F, et al. A pilot in vivo investigation of peripheral nerve damage in multiple sclerosis using magnetisation transfer ratio. ISMRM 2020, 1401.
Figures
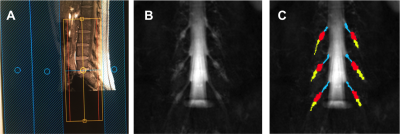
Figure 1. A) Prescription
of the 3D SHINKEI and MTR sequences for imaging the lumbar plexus in the
coronal plane: B) Example image obtained using the 3D SHINKEI at the level of
the lumbar plexus (L2-L4 segments shown); C) Manual segmentation
of the lumbar segments with separate binary masks created for the
preganglionic, ganglionic and postganglionic regions shown in blue, red,
yellow, respectively.
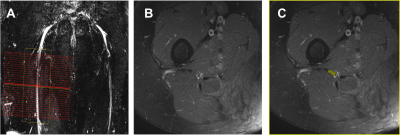
Figure 2. A) Prescription
of the high-resolution
fat-suppressed T2-weighted and MTR sequences for imaging the sciatic
nerve in the axial plane; B) Example image obtained using the high-resolution
fat-suppressed T2-weighted sequence; C) Manual segmentation of the sciatic
nerve (binary mask shown in yellow).
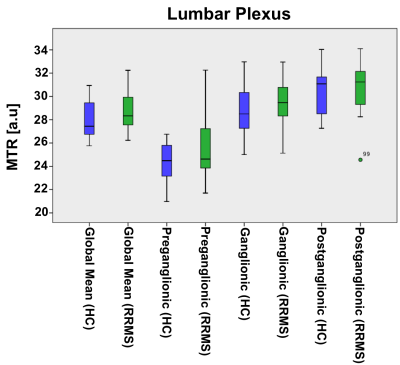
Figure 3. Distribution of
MTR values in the lumbar plexus (all lumbar segments combined i.e. global mean)
of healthy controls (blue) and people with RRMS (green), and also results from
separate measurements in the preganglionic, ganglionic and postganglionic
regions; no statistically significant differences between the groups were
identified in global or regional MTR measurements.
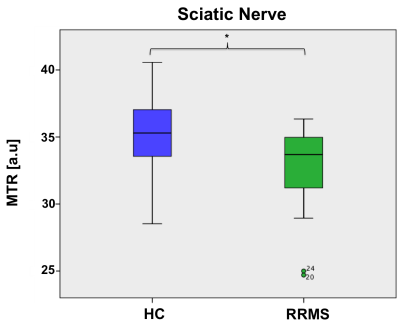
Figure 4. Distribution of
MTR values in the sciatic nerve of healthy controls, mean (±SD) 35.1 (±3.9) and
people with RRMS (32.4 ± 4.4) showing a statistically significant difference
(p=0.027).
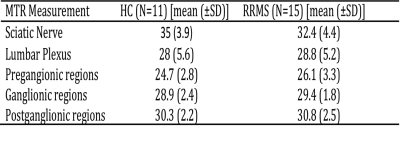
Figure 5. Summary of all
MTR measurements in HCs and people with RRMS at each anatomical location.