2800
Ultrahigh-b radial Diffusion Weighted Imaging (UHb-rDWI) of Wild Type and Shiverer Mouse Spines
Kyle Jeong1, You-Jung Lee1, Suk-Keu Yeom2, Noel Carlson3, Lubdha Shah4, John Rose5, and Eun-Kee Jeong4
1Utah Center for Advanced Imaging research, University of Utah, Salt Lake City, UT, United States, 2Radiology, Korea University Ansan Hospital, Ansan, Korea, Republic of, 3GRECC, Veteran Affairs, Salt Lake City, UT, United States, 4Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 5Neuroimmunology Division, University of Utah, Salt Lake City, UT, United States
1Utah Center for Advanced Imaging research, University of Utah, Salt Lake City, UT, United States, 2Radiology, Korea University Ansan Hospital, Ansan, Korea, Republic of, 3GRECC, Veteran Affairs, Salt Lake City, UT, United States, 4Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 5Neuroimmunology Division, University of Utah, Salt Lake City, UT, United States
Synopsis
Ultrahigh-b radial DWI (UHb-rDWI) is an MRI technique that can quantitatively evaluate the MS lesions with respect to degrees of demyelination and axonal damage and/or loss. With the current lack of sensitive diagnostic imaging for grading cervical spinal cord (CSC) injury and repair, our UHb-rDWI can potentially serve as a powerful tool to observe cervical spinal cord once validated through animal studies. Therefore, the main objective of this study was to evaluate mouse spinal cords with and without demyelination using UHb-rDWI and immunohistochemical analyses to authenticate the reliability and reproducibility of UHb-rDWI.
Introduction
CSC is extremely compact where all motor and sensory tracts with 1 ~ 2 million axons pass through a small cross-sectional area of roughly 1.0 cm2 at C3 vertebrae for example 1. Hence, any CSC damage from demyelinating diseases, such as MS, compression or trauma may lead to severe disability. Unfortunately, clinicians currently lack sensitive diagnostic imaging for grading CSC injury and repair and rely on subjective and flawed imaging diagnostic tests 2. For instance, T2-weighted MRI, routinely used to evaluate spinal cord pathology, is limited in detecting information regarding presence demyelination, axonal loss or remyelination. Thus, a more sensitive technique is urgently greatly desiredd to characterize CSC injury in spinal cord injury (SCI), clinically isolated syndrome (CIS), multiple sclerosis (MS) and diseases causing myelitis or myelopathy. Fortunately, our findings suggest that UHb-rDWI could serve as a powerful tool to quantitatively evaluate disease progression and monitor treatment responses in various CSC diseases. In this study, we will present our UHb-rDWI evaluations correlated with immunohistochemical analyses of mouse spinal cords, with and without demyelination, to validate UHb-rDWI.Methods
All MRI experiments were performed on mice spine columns, placed in a homemade Alderman-Grant Resonator RF coil, using 3D multi-shot DW-stimulated-EPI (3D ms-DWSTEPI) on a 7T small-bore animal MRI system (Bruker BioSpin, Karlsruhe, Germany). The following Imaging parameters were used: TR 500 ms, TE 32 ms, ETL 20 (8 shots), receiver bandwidth 2600 Hz/px, acquisition matrix 192x160x24, number of signal average 28, in-pane spatial resolution 75x75 μm2 with 1.0-mm slice thickness. The diffusion gradient was applied with duration δ = 5 ms and separation Δ = 100 ms, perpendicular to the cord for UHb-rDWI with ten b-values = 165 ~ 42890 s/mm2 and parallel to the cord for UHb-aDWI with six b-values = 165 ~ 2210 s/mm2, respectively. Diffusion-weighting was varied by varying the amplitude of the diffusion gradient with fixed duration and separation. All spinal cords fixed in 4% formaldehyde in PBS for 24 hours and subsequently transferred to PBS were prepared for histology after MRI. 2-mm spinal cord segments were removed from the upper thoracic columns for electron microscopy (EM). For EM, coronal specimen segments were fixed and stained with OsO4 for 24 hrs, embedded into plastic blocks, and sliced into 70 ~ 80-nm thick segments. EM analyses were performed using a Transmission Electron Microscopy (TEM, FEI Technai 12) in the NanoFab core EM laboratory of University of Utah.Results and Discussions
UHb-rDWI were analyzed for ROIs at anterior-doorssal (Fig. 1(d~f)), posterior-dorsal (Fig. 1(d~f)), and lateral WM tract (Fig. 1(g~i)). Signal-b curve was fit to the exponential function for each animal and low-b and high-b diffusion coefficients (DL, DH) and D values were averaged over two adjacent slices of 6 WT and 2 shivered mice, respectively. For all three regions, WT data fit well to double-exponential function and shiverer data fit to single-exponential function. At anterior-dorsal column, DL and DH of the WT cord were estimated (0.593±0.310, 0.019±0.006)x10-3mm2/s, while that of shiverer cord is D = (0.162±0.011)x10-3 mm2/s. At posterior-dorsal column, they are estimated as (0.434±0.190, 0.015±0.004)x10 mm2/s and D = (0.142±0.014)x10-3 mm2/s for WT and shiverer cords, respectively. And those for lateral columns were (0.387±0.225, 0.020±0.004) x10-3 mm2/s and D = (0.164±0.020) x10-3 mm2/s for WT and shiverer cords, respectively. D of the shiverer mice is about an order of magnitude larger than DH in all three regions. EM of shiverer and WT mice at the dorsal and lateral columns are presented in Figs. (2, 3), respectively. Myelin is thinner in the shiverer (Fig. 2(b, d), Fig. 3(b, d)) than WT (Fig. 2(a, c), Fig. 3(a,c)) cords. This observation matches the report in which shiverer myelin is said to have only a few lipid-bilayers 3, which induces a significant increase in water permeability at the membrane with , that consequently results in an increase in DH. Therefore, these histologic data are consistent with UHb-DWI result and confirms that the DH is a definitebiomarker with high specificity for quantitative evaluation of the myelination in white-matter tract.Conclusion
Genetically engineered myelin-deficit (shiverer) and WT mouse species were successfully used to study the efficacy of UHb-rDWI in quantitatively characterizing CSC with respect to degrees of demyelination. UHb-rDWI signal-b curves illustrated significant difference in DH between shiver and WT specimens. UHb-rDWI can be used to quantitatively evaluate and monitor CSC disease progression and treatment response.Acknowledgements
This work was funded by NIH R56R01 NS106097-01A1 (E.K. Jeong) and the National Research Foundation (NRF) of Korea (NRF-2018R1C1B5084960) (Yeum), as well as support from Alice and Kevin Steiner.References
- Saliani, A. et al. Axon and Myelin Morphology in Animal and Human Spinal Cord. Front. Neuroanat. 11, 129 (2017).
- Bot, J. C. & Barkhof, F. Spinal-Cord MRI in Multiple Sclerosis: Conventional and Nonconventional MR Techniques. Neuroimaging Clin. N. Am. 19, 81–99 (2009).
- Inoue, Y., Inoue, K., Terashima, T., Mikoshiba, K. & Tsukada, Y. Developmental changes of oligodendroglia in the posterior funiculus of ‘Shiverer’ mutant mouse spinal cord, with special reference to myelin formation. Anat. Embryol. (Berl). (1983). doi:10.1007/BF00315814
Figures
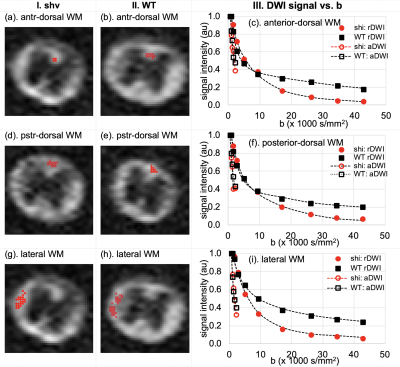
Fig. 1. DWIs of b=9380 s/mm2 of (col I) shiverer and (col II) WT mice spinal cords, and signal-b curves of (c) anterior-dorsal and (f) posterior-dorsal column, and (i) lateral white-matter tracts up to b = 42,890 s/mm2 for UHb-rDWI and 2210 s/mm2 for UHb-aDWI. Signals were averaged over two nearby slices of two shiverer (red) and two adjacent slices of six WT (black) mouse spinal cords.
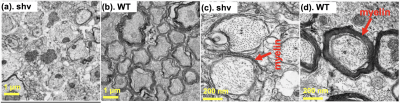
Fig. 2. EM images of dorsal (sensory) column of (a, c) shiverer and (b, d) WT mice spinal cords in two different magnifications (a, b) x1,100 and (c, d) x11,000. In this EM picture, the myelin sheaths of the shiverer mouse is less numbered and looser than that of the wild-type mouse.

Fig. 3. EM images of lateral (motor) column of (a, c) shiverer and (b, d) WT mice spinal cords in two different magnifications (a, b) x2,200 and (c, d) x11,000. The approximate number of myelin sheaths, indicated by red arrows in (c, d), is 2-3 layers in shiverer mice, compared to 10-20 layers in WT mice.