2799
Blood-brain barrier permeability changes in multiple sclerosis during alemtuzumab treatment1Dept. Clinical Physiology, Rigshospitalet, Glostrup, Denmark, 2Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark, 3Dept. of Neurology, Rigshospitalet, Glostrup, Denmark
Synopsis
Ineffective disease control in multiple sclerosis leads to permanent disability, and biomarkers for early detection of inadequate treatment response are needed. The potential of the patlak derived influx constant (Ki) as a biomarker was investigated in fifteen relapsing-remitting multiple sclerosis patients undergoing alemtuzumab treatment. The subjects underwent dynamic contrast enhanced MRI and 3DT1-weighted scans to assess Ki in grey and white matter before and after treatment. The treatment associated change in Ki in grey matter predicted disease activity within two years. Treatment associated changes in Ki may be used as a biomarker of treatment efficacy.
Introduction
Deciding the optimal treatment for multiple sclerosis (MS) is challenging since ineffective disease control may lead to permanent disability, while highly effective treatments can have serious adverse effects. Further, the timing of treatment is important since early disease control reduces the risk of permanent disability1. Therefore, tools for early detection of inadequate treatment response and decision support are needed. The blood-brain barrier (BBB) is compromised in multiple sclerosis2, and the permeability of the BBB has previously been shown to predict treatment response to natalizumab and fingolimod, which are second line MS treatments3. Therefore, BBB integrity measured by MRI was investigated as a marker of treatment efficacy for alemtuzumab.Methods
Fifteen patients with relapsing-remitting multiple sclerosis initiating alemtuzumab treatment underwent dynamic contrast enhanced (DCE) MRI scans before and 6, 12 and 18 months after treatment. The first course was administered after the baseline scan, and the second course after the 12-month scan. BBB permeability in grey and white matter was assessed as the Patlak derived influx constant (Ki) by DCE-MRI as previously described4. DCE repetition time (TR) was 3.82 ms and echotime (TE) 1.9 ms with a time resolution of 1.25 s. Ki values from grey and white matter were derived from 3D T1w MRI by automatic tissue segmentation using FSL. Contrast enhancing lesions were removed from the tissue volume by use of a post contrast T1 weighted sequence. Treatment response according to no evidence of disease activity-3 (NEDA-3) criteria, expanded disability status scale (EDSS) score, methylprednisolone use and leukocyte counts were collected from medical records at two years from treatment initiation. Welch t-test, ROC curve analysis and a linear mixed model with subject as random effect and disease status and time as fixed effects were calculated using R with the ROCR, nlme and lattice packages.Results
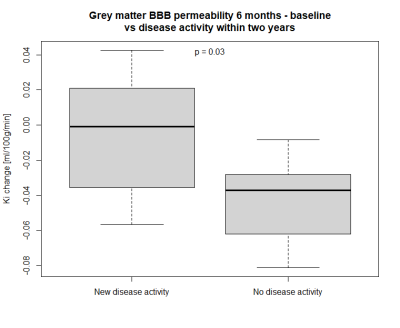
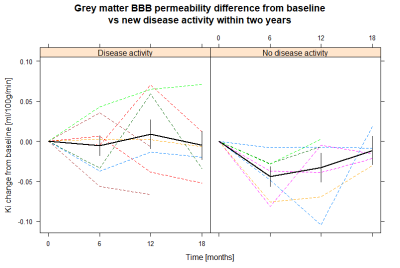
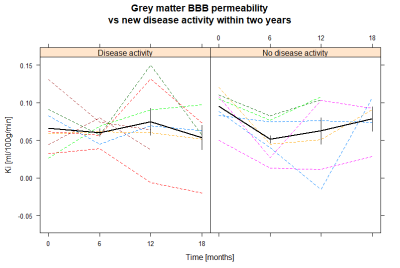
Patients who maintained NEDA status at two years after treatment initiation had a significantly larger decrease in BBB permeability in grey matter between baseline and six months follow-up than those who experienced disease activity (mean difference –0.038 ml/100g/min, 95% confidence interval -0.073;-0.004, p = 0.03). For white matter there was no significant difference between the two groups (p = 0.9). ROC curve analysis for change in grey matter permeability showed a trend towards that a cut-off at -0.036 ml/100g/min predicted loss of NEDA status with 86% sensitivity and 63% specificity (AUC 0.79, p = 0.07). There was no significant difference for the 12-month or the 18-month course of BBB permeability changes between the two groups. Age, gender and EDSS were similar between the two MS groups. One subject in the group with new disease activity received methylprednisolone less than 60 days prior to the initial scan, whereas this was true for two subjects in the NEDA group.Discussion
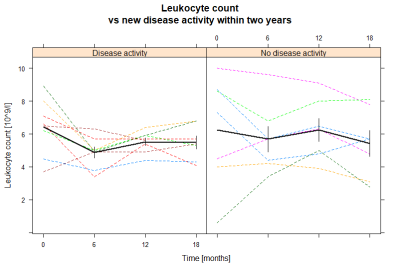
Our results indicate that the treatment associated change in BBB permeability could be used as a surveillance tool of treatment response. BBB permeability in grey matter decreases from baseline to six months, after the initial alemtuzumab course, but not from 12 months to 18 months, after the second course. This is different from leukocyte counts which decrease after each course. However, the leukocyte count does not fully recover before the second course which possibly could explain why there is no observed drop in BBB permeability after the second course5. The fact that methylprednisolone, which decreases the permeability of the BBB, was administered to two subjects in the NEDA group but only one subject in the disease activity group supports the finding that a decrease in BBB permeability indeed is correlated to alemtuzumab treatment response, and is not simply an effect of methylprednisolone. DCE-MRI has previously been used to examine treatment response, but this is the first time the treatment associated change in BBB permeability is reported.Conclusion
The BBB permeability as measured by the influx constant (Ki) by DCE-MRI decreases during treatment with alemtuzumab in relapsing-remitting multiple sclerosis. Further, the change in BBB permeability after treatment initiation may be used as a predictor of long-term treatment efficacy. The magnitude of change in BBB permeability after alemtuzumab initiation predicted treatment efficacy at two years, validating previous research indicating that BBB permeability may be used as a marker of treatment response in relapsing-remitting multiple sclerosis.This investigator initiated study received funding from Sanofi and is registered at ClinicalTrials.gov with study number NCT03193086.
Acknowledgements
No acknowledgement found.References
1. Harding, K. et al. Clinical Outcomes of Escalation vs Early Intensive Disease-Modifying Therapy in Patients with Multiple Sclerosis. JAMA Neurology 76, 536–541 (2019).
2. Ortiz, G. G. et al. Role of the Blood-Brain Barrier in Multiple Sclerosis. Archives of Medical Research vol. 45 687–697 (2014).
3. Cramer, S. P. et al. Permeability of the blood–brain barrier predicts no evidence of disease activity at 2 years after natalizumab or fingolimod treatment in relapsing–remitting multiple sclerosis. Annals of Neurology 83, 902–914 (2018).
4. Larsson, H. B. W., Courivaud, F., Rostrup, E. & Hansen, A. E. Measurement of brain perfusion, blood volume, and blood-brain barrier permeability, using dynamic contrast-enhanced T1-weighted MRI at 3 tesla. Magnetic Resonance in Medicine 62, 1270–1281 (2009).
5. Hersh, C. M. & Cohen, J. A. Alemtuzumab for the treatment of relapsing-remitting multiple sclerosis. Immunotherapy 6, 249–259 (2014).
Figures