2794
Diffusely abnormal white matter in clinically isolated syndrome is associated with parenchymal loss and elevated neurofilament levels1Radiology, University of British Columbia, Vancouver, BC, Canada, 2Physics and Astronomy, University of British Columbia, Vancouver, BC, Canada, 3International Collaboration on Repair Discoveries (ICORD), University of British Columbia, Vancouver, BC, Canada, 4Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada, 5Medicine, University of British Columbia, Vancouver, BC, Canada
Synopsis
We characterized the frequency of diffusely abnormal white matter (DAWM) in a broad cohort of multiple sclerosis (MS) participants. 35% of clinically isolated syndrome (CIS) and ~60% of MS participants had DAWM. CIS with DAWM had smaller cortical thickness, higher lesion load and higher concentration of neurofilament light chain compared to CIS without DAWM. DAWM may be useful in identifying brains at risk of injury but only in the CIS population when lesion load is low. Longitudinal studies are warranted.
Introduction
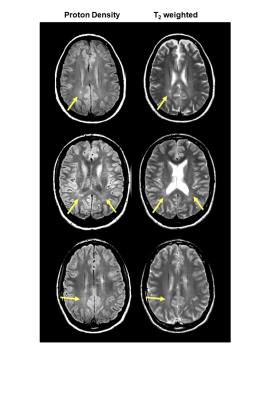
Multiple sclerosis (MS) is characterised by focal areas of demyelination within the CNS termed lesions. In some brains, diffuse areas of increased signal on proton density (PD) and T2-weighted images may be visible – this phenomena is known as diffusely abnormal white matter (DAWM, Figure 1).1,2 DAWM has ill-defined borders and similar signal intensity to grey matter on PD/T2 scans, but is found within white matter -- predominantly periventricular regions and the centrum semiovale.3 Pathologic examination of DAWM in MS demonstrates blood-brain barrier breakdown,4 extensive loss of myelin phospholipids, and variable degrees of axonal loss.5 The degree of these changes is intermediate to those found in normal appearing white matter and MS plaques.6 While histology studies of DAWM describe specific tissue changes, post-mortem work typically only captures information from the very end-stages of disease. Blood-based biomarkers may be able to provide information about disease processes along the course of MS development. One such candidate biomarker is neurofilament light chain (NfL), a neuraxonal-specific protein7 which is increased in the blood after CNS tissue damage.8 NfL has not been studied in MS participants with (DAWM+) and without (DAWM–) DAWM, and the frequency of DAWM, alongside the MR volumetrics in DAWM+/– brains have not been well documented.Objectives
We examined the frequency of DAWM in a broad cohort of MS participants. Brain, lesion and grey matter volume, cortical thickness and NfL were compared between participants with and without DAWM.Methods
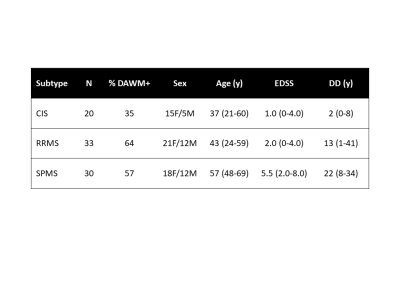
Subjects and MR Experiments: 83 participants with clinically isolated syndrome (n=20), relapsing remitting MS (RRMS, n=33) and secondary progressive MS (SPMS, n=30) were scanned at 3T (Philips Achieva, demographics in Figure 2). Sequences included PD/T2-weighted (TR/TE1/TE2=2900/8.42/80ms, 1x1x3mm3), and 3DT1-MPRAGE (TR/TE/TI=3000/3.5/926ms, 1x1x1mm3). The CIS/MS participants had blood collected on the same day as MR experiments. Blood from 35 healthy age and sex-matched controls was also collected.Data Analysis: Two experienced MRI researchers working independently identified DAWM on PD and T2-weighted scans as regions of white matter that were iso-intense to grey matter, present on at least two consecutive scan slices, and >10mm in diameter. A senior radiologist reviewed scans with DAWM identification disagreement and a final determination was obtained by consensus.
Normalised brain volume was calculated using in-house software.9 Cortical thickness was determined with ANTs.10 Thalamic and deep grey matter (GM, caudate + putamen + thalamus + pallidum) volumes were calculated with FIRST.11 Lesions were automatically segmented using seed points.12 Serum NfL was quantified by single molecule array (SIMOA) technology (Quanterix). For NfL, controls were subdivided so that age and sex were matched to each CIS/MS subtype. A percentage difference between each participant and their matched control was calculated to account for age and sex effects in NfL. Measurements were compared using t-tests (p<0.05) for this exploratory study.
Results
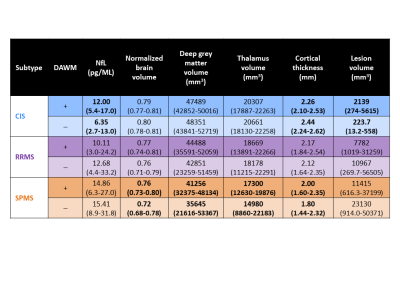
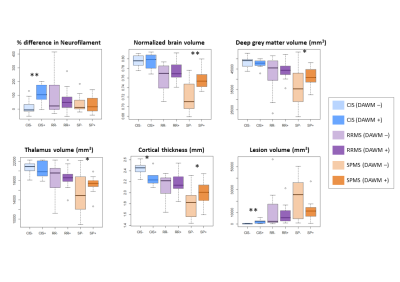
35% of CIS and ~60% of MS participants had DAWM (Figure 2). DAWM+ CIS had smaller cortical thickness (p=0.006), higher lesion load (p=0.001) and higher NfL (p=0.002) compared to DAWM– CIS (Figure 3 and 4). DAWM+ SPMS participants showed the opposite trend with larger normalised brain (p=0.001) and grey matter volumes (p=0.05), and larger cortical thickness (p=0.04) (Figure 3 and 4). No significant differences were found between RRMS with and without DAWM. RRMS and SPMS NfL was similar between DAWM+ and DAWM– (Figure 3 and 4).Discussion
DAWM was observed after a single demyelinating event in approximately one third of CIS participants, in line with a previous study where 27% of CIS participants had DAWM.13 57-64% of clinically definite MS participants demonstrated DAWM, much higher than previous reports of ~25% at 1.5T.1 Our use of 3T MRI likely resulted in better detection of subtle signal changes in the white matter and a higher reporting of DAWM.DAWM+ CIS participants had a higher lesion load than DAWM– CIS, but overall CIS lesion volume was much smaller than RRMS and SPMS. In MS, lesions may obscure the identification of DAWM, resulting in the trend of higher lesion load in DAWM– RRMS and SPMS participants.
The increased NfL in DAWM+ CIS suggest there may be injury in these areas leading to other negative brain health markers (i.e. smaller cortical thickness, more lesions). In SPMS, the DAWM– participants had more lesions and smaller brain and grey matter volumes, Different mechanism of injury in SPMS and CIS may be underlying these observations. In CIS, DAWM could be areas of inflammation which cause some of the early injury in the disease. In SPMS, neurodegeneration becomes the dominant mechanism of injury; the inflammation within DAWM may no longer be as important or DAWM+ status may paradoxically indicate less overall tissue loss because it is more evident with smaller lesion volume. Abnormalities in myelin phospholipids demonstrated by histology6 could indicate that DAWM identifies a unique subgroup of patients with MS with an inherent defect in myelin. This potentially makes their white matter more vulnerable to inflammation.
Conclusion
This is the first report examining NfL in CIS and MS participants with and without DAWM. DAWM may be useful in identifying brains at risk of injury but only in the CIS population when lesion load is low. Longitudinal studies are warranted.Acknowledgements
We sincerely thank all study participants, the staff at the UBC MS clinic and the MR technologists at the University of British Columbia MRI Research Centre. This work was funded by the Multiple Sclerosis Society of Canada.References
1. Zhao GJ, Li DKB, Cheng Y, et al., MRI dirty-appearing white matter in MS. Neurology, 2000. 54 (suppl 3): p. A121.
2. West J, Aalto A, Tisell A, et al., Normal appearing and diffusely abnormal white matter in patients with multiple sclerosis assessed with quantitative MR. PLoS ONE, 2014. 9(4): p. e95161.
3. Yulin G, Grossman RI, Babb JS, et al. Dirty-appearing white matter in multiple sclerosis: volumetric MR imaging and magnetization transfer ration histogram analysis. AJNR Am J Neuroradiol 2003;24:1935-1940.
4. Vos CMP, Geurts JJG, Montagne L, et al. Blood–brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiol Dis 2005;20:953-960.
5. Laule C, Pavlova V, Leung E, Zhao G, MacKay AL, Kozlowski P, Traboulsee A, Li DKB, Moore GRW. Diffusely abnormal white matter in multiple sclerosis: further histologic studies provide evidence for a primary lipid abnormality with neurodegeneration. J Neuropathol Exp Neurol. 2013 Jan;72(1):42-52.
6. Ropele S, Strasser-Fuchs S, Augustin M, et al. A comparison of magnetization transfer ratio, magnetization transfer rate, and the native relaxation time of water protons related to relapsing–remitting multiple sclerosis. Am J Neuroradiol 2000;21:1885-1891.
7. Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol. 2017;9(4):a018309.
8. Thebault S, Booth RA, Freedman MS. Blood Neurofilament Light Chain: The Neurologist's Troponin? Biomedicines. 2020;8(11):523.
9. Wicks E, Chiu JPC, Tang LYW, Lam K, Riddehough A, Li DKB, Traboulsee A, Tam R. Automatic Computation of Normalized Brain Volume on 3D T1-Weighted MRI Scans Without Registration to Standard Space, ISMRM. 2015, 3750.
10. Das SR, Avants BB, Grossman M, Gee JC. Registration based cortical thickness measurement. Neuroimage. 2009;45:867-79.
11. Patenaude B, Smith SM, Kennedy D, Jenkinson M. A Bayesian Model of Shape and Appearance for Subcortical Brain NeuroImage. 2011; 56: 907-922.
12. McAusland J, Tam RC, Wong E, et al. Optimizing the Use of Radiologist Seed Points for Improved Multiple Sclerosis Lesion Segmentation. IEEE Trans Biomed Eng. 2010;57:2689–2698.
13. Laule C, Lee J, Zhao G, White R, Vavasour IM, Tam R, Riddehough A, Traboulsee AL, Metz L, Li DKB. Prevalence of diffusely abnormal white matter in individuals with clinically isolated syndromes suggestive of multiple sclerosis. 25th Annual Meeting of the International Society of Magnetic Resonance in Medicine, Honolulu, USA, April 22-28, 2017
Figures