2793
Hippocampal subfield volumes relate to future cognitive performance in Multiple Sclerosis1The Cleveland Clinic, Cleveland, OH, United States
Synopsis
Cognitive dysfunction is a common symptom of Multiple Sclerosis (MS), and patients would benefit from a measure that estimates their risk of future decline. Previous work suggests that hippocampal subfield volumes may have value as a predictive measure. Using 7 tesla MRI, we measured hippocampal volumes in 77 participants with MS. We found relationships between subfield volumes and future cognitive performance. These relationships were driven by relapse remitting MS patients, although the secondary progressive MS group did not show clear differences in the relationship patterns.
Introduction
Hippocampal atrophy is common in patients with Multiple Sclerosis (MS).1 Region demyelination in MS has been reported,2 particularly in cornu ammonis 1 region (CA1),3 and CA1 atrophy has been related to performance on a number of neuropsychological tests.1,4 Our previously published work showed cross-sectional relationships between hippocampal volume and episodic memory, particularly on the left.5 These relationships suggest that hippocampal volumes may have value as a predictor of future cognitive decline. Here we present results from a large longitudinal study of neuroimaging in MS, focusing on the relationship of hippocampal volume to cognitive performance and looking at differences between relapse remitting (RR) and secondary progressive (SP) MS.Methods
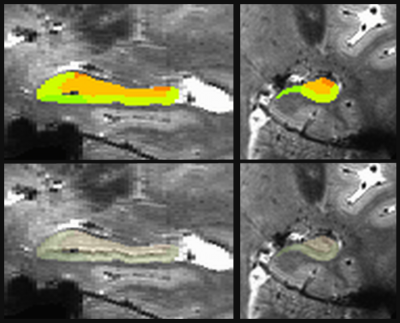
Participants were 77 patients with MS (mean age 51.2 ± 8.1; 19 males; median EDSS 3.5; 62 RR; 15 SP). All participants had baseline MRI scanning and cognitive testing (TP1), with 58 participants returning for cognitive testing after one year (TP2). Cognitive tests included measures of verbal and spatial episodic memory, verbal fluency, and processing speed. MRI scans were acquired on a Siemens 7T Magnetom with SC72 gradient (Siemens Medical Solutions, Erlangen) using a single-Tx/32-channel Rx head coil (Nova Medical). Scans included a whole-brain anatomical MP2RAGE (0.75mm3 isotropic voxel size; WIP 900) and a high-resolution GRE scan focusing on the hippocampus (0.5x1.0x0.5mm3, coronal acquisition). Using the high-resolution GRE scan and the MP2RAGE, left and right hippocampi were automatically segmented for each subject using the Automated Segmentation of Hippocampal Subfields software,6 optimized for 7 tesla (Figure 1).7 Volumes of bilateral subiculum (SUB), CA1, dentate gyrus (DG), total CA (CAt; CA1+CA2+CA3), and total hippocampus were calculated and corrected for intracranial volume. Separately for RR and SP participants, TP1 volumes were compared to cognitive performance at TPs 1 and 2.Results
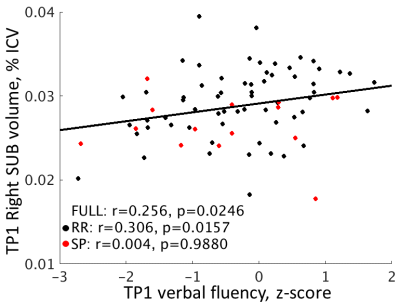
Hippocampal volumes were not significantly different between those with RR and SP MS.TP1 volume relationships to TP1 cognition. In the full sample (n = 77), visual spatial episodic memory was positively related to bilateral DG volumes (p < 0.025). Right SUB volume was related to verbal episodic memory (r = 0.364, p = 0.0011) and verbal fluency (r = 0.256, p = 0.0246; Figure 2). The relationships to visual spatial episodic memory and verbal fluency (p < 0.02) were driven by the RR group (n = 62). The relationship to verbal episodic memory was only weakly significant in the separate groups (p < 0.06).
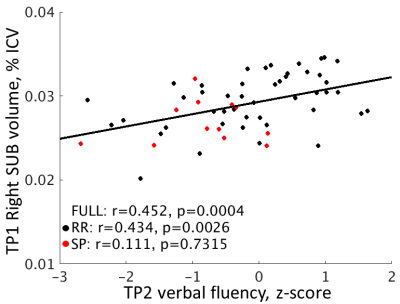
TP1 volume relationships to TP2 cognition. In the full sample (n = 58), the only relationship to cognition was between right SUB volume and verbal fluency (r = 0.452, p = 0.0004; Figure 3). This relationship was only significant in the RR group (n = 46; r = 0.434, p = 0.0026). Visual spatial episodic memory was positively related to right CA1, CAt, and total hippocampal volume in the RR group (p < 0.035). No relationships were independently significant in the SP group (n = 12).
Discussion
In contrast to previous work, we did not find preferential relationships between CA1 and cognitive measures in MS. Bilateral DG was related to visual spatial episodic memory, but only right SUB volume was related to verbal episodic memory. Previous work found bilateral relationships between hippocampal volume and episodic memory,1 though our work found significant relationships on the left only.5 The SP and RR groups did not show differences in any subfield volumes. Although the RR group drove the majority of cognitive relationships, the lack of significant relationships in the SP group may have been due to the small sample size. The relationship of subfield volumes to cognitive function measured after one year suggests that hippocampal volumes may be useful in the prediction of cognitive decline. Additional work will look at the change cognitive measures measured over a longer period.Acknowledgements
This work was supported by the Department of Defense (MS150097). We thank Siemens Healthineer Tobias Kober for use of WIP944.References
1. Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131(4):1134-41.
2. Geurts JJ, Bö L, Roosendaal SD, Hazes T, Daniëls R, Barkhof F, et al. Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol. 2007;66(9):819-27.
3. Papadopoulos D, Dukes S, Patel R, Nicholas R, Vora A, Reynolds R. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathology. 2009;19(2):238-53.
4. Longoni G, Rocca MA, Pagani E, Riccitelli GC, Colombo B, Rodegher M, et al. Deficits in memory and visuospatial learning correlate with regional hippocampal atrophy in MS. Brain Struct Funct. 2015;220:435-44.
5. Koenig KA, Sakaie KE, Lowe MJ, Lin J, Stone L, Bermel RA, et al. Hippocampal Volume Is Related to Cognitive Decline and Fornicial Diffusion Measures in Multiple Sclerosis. MRI. 2014;32(4):354-8.
6. Yushkevich PA, Pluta J, Wang H, Ding SL, Xie L, Gertje E, et al. Automated Volumetry and Regional Thickness Analysis of Hippocampal Subfields and Medial Temporal Cortical Structures in Mild Cognitive Impairment. Human Brain Mapping 2014; 36(1): 258-87.
7. Wisse LEM, Kuijf HJ, Honingh AM, Wang H, Pluta JB, Das SR, et al. Automated Hippocampal Subfield Segmentation at 7T MRI. American Journal of Neuroradiology 2016; 37(6): 1050-7.
Figures