2790
Sex-based differences in tissue microstructure in multiple sclerosis detected using multi-model diffusion MRI
Olayinka Adeoluwa Oladosu1, Cayden Murray2, Syed Rizvi2, Mariana Bento3,4, G Bruce Pike3,4, and Yunyan Zhang3,4
1Neuroscience, University of Calgary, Calgary, AB, Canada, 2University of Calgary, Calgary, AB, Canada, 3Radiology, University of Calgary, Calgary, AB, Canada, 4Clinical Neurosciences, University of Calgary, Calgary, AB, Canada
1Neuroscience, University of Calgary, Calgary, AB, Canada, 2University of Calgary, Calgary, AB, Canada, 3Radiology, University of Calgary, Calgary, AB, Canada, 4Clinical Neurosciences, University of Calgary, Calgary, AB, Canada
Synopsis
Sex difference in multiple sclerosis (MS) prevalence is well recognized but impacts on tissue microstructure and its variations with respect to disease modifying therapies (DMTs) remains unclear. Here we investigated sex-based differences in lesion microstructure and its variations with therapeutic approaches in 52 relapsing-remitting MS patients using diffusion tensor, compartment, and orientation models. We found that men had greater myelin and axonal damage than women in MS lesions, and there was less axonal damage in women taking oral versus injectable DMTs. These results may support further investigations of sex-driven differences in disease monitoring and treatment choices to promote personalized medicine.
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating and neurodegenerative disease of the central nervous system characterized by complex injury to both myelin and axons. MS impacts women 3 times more than men; however, men often have a much more aggressive disease course than women1,2. While the mechanisms remain unclear3, these differences between women and men may impact their tissue properties4 and their responses to disease modifying therapies (DMTs). In this study, our goal was to investigate how tissue microstructure was different between women and men and whether and how it varies between injectable and oral DMTs in each sex, based on multi-model analysis of diffusion MRI in MS lesions. These analyses may have important implications in disease monitoring and management particularly for those with relapsing remitting MS (RRMS) where various DMTs are available.Methods
52 patients with relapsing-remitting MS were scanned in a 3T MRI system using both anatomical and diffusion imaging. The imaging protocol included T1-weighted MRI acquired with a fast-spoiled gradient echo BRAVO sequence using TR/TE = 6.7/2.9 ms; matrix = 256x256, FOV = 25.6x25.6 cm, slice thickness = 1 mm, and FLAIR MRI obtained with a spin echo inversion recovery sequence using TR/TE = 7000/127 ms; matrix = 224x224, FOV = 24x24 cm, slice thickness = 1mm. Diffusion MRI was acquired with spin-echo echo planar imaging using TR/TE = 8000/61 ms; matrix = 120x120, FOV = 24x24cm, slice thickness=2mm, b0=3, b-value=1000, 45 directions.We performed lesion segmentation on FLAIR MRIs that were co-registered to T1-weighted images. Using diffusion MRI processed for denoising, Gibbs ringing, eddy currents, and susceptibility distortion, we calculated whole-brain DTI measures including fractional anisotropy, axial, radial, and mean diffusivity, and sub-voxel based compartment measures by treating the diffusion data as single-shell HARDI (high angular resolution diffusion imaging), including intracellular volume fraction (ICVF), diameter, and density, using the AMICO-ActiveAx model5,6. Further, we computed additional orientational measures including orientation dispersion index (ODI), orientation distribution function (ODF) energy, fiber density, and fiber termination indices (FDi/FTi)), from AMICO and DTI tractography models7,8. Based on measurements in lesions and the contralateral normal appearing white matter (NAWM) areas, we calculated a normalized lesion parameter named asymmetry $$$(lesion - NAWM)/(lesion + NAWM)$$$, per region of interest (ROI). Positive asymmetry values indicate higher measures in lesions than NAWM, and negative values indicate higher measures in NAWM than lesions. Values closer to zero point to smaller differences between the two tissues.
Fixed effects models were constructed to assess differences between women and men in tissue microstructure, then injectable (DMT1) versus oral (DMT2) DMTs in treatment analysis compared within each sex. Significance was established with p<0.05.
Results
Of the 52 RRMS patients, there were 36 women (mean age = 44.9 years, range = 27-58 years) and 16 men (mean age = 43.0 years, range = 23-57 years). The treatment included 7 different brands of first-line DMTs with both injectable (interferon and glatiramer acetate, n=13) and oral therapies (fingolimod, teriflunomide, and dimethyl fumarate, n=39).In tissue microstructure analysis, fractional anisotropy, axonal density, and ICVF were significantly higher in lesion asymmetry (p<0.05) in women than men. Axonal diameter, mean diffusivity, radial diffusivity, and ODF energy (p<0.05) asymmetry were significantly lower in women than men. ODI, FDi, and FTi asymmetry showed no significant differences between sex (Figure 1-2).
Women treated by injectable DMTs had significantly lower axial diffusivity asymmetry than women taking oral DMTs (Figure 3). Women taking oral DMTs showed asymmetries closer to zero (more similar lesion vs NAWM values) in diameter, axonal density, fractional anisotropy, and ICVF measures(p<0.05) than women taking injectable DMTs (Figures 3-4). FDi (p<0.05), ODF entropy(p<0.0001), and ODI(p<0.05) asymmetries were significantly higher with lesions values increased compared to NAWM for women taking oral DMTs than those taking injectable DMTs (Figure 5).
Discussion
We observed differences in severity of microstructure pathology and its patterns according to injectable versus oral DMTs associated with patient sex using multi-model diffusion MRI measures. This study found better microstructure integrity in women than men suggesting lesser lesion damage. This finding aligns with the literature showing that men typically have a more aggressive course of MS4,9. Various factors may play a role, including hormone effects on immune and nervous systems3. Comparing tissue microstructure between injectable and oral DMT groups, significant differences were identified in women where oral DMTs were associated with smaller lesion-NAWM differences, primarily in measures of axon integrity including density, diameter, and coherence. This suggests a greater axon-protective effect by oral DMTs in women. Literature identifies greater compliance and effectiveness of oral over injectable DMTs which may partially account for the benefits of oral DMTs10–12. A limitation is that women and men are not matched in all parameters such as age and disease duration, which may impact the outcomes.Conclusion
There seem to be sex differences in MS tissue microstructure such that myelin and axon damage are more severe in men as detected by diffusion MRI measures. Differences were also detected in tissue microstructure compared between treatment types in which women taking oral DMTs exhibited greater axonal sparing according to diffusion models. These findings may aid development of clinical approaches designed to optimize treatment outcomes according to patient sex.Acknowledgements
We thank the graduate studentship funding support of the Alberta Graduate Education Scholarship. We also thank the funding support from the MS Society of Canada, Natural Sciences and Engineering Council of Canada (NSERC), and Alberta Innovates Health Solutions.References
- Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Prim 2018; 4: 43.
- Ribbons KA, McElduff P, Boz C, et al. Male sex is independently associated with faster disability accumulation in relapse-onset MS but not in primary progressive MS. PLoS One 2015; 10: 1–11.
- Golden LC, Voskuhl R. The importance of studying sex differences in disease: The example of multiple sclerosis. J Neurosci Res 2017; 95: 633–643.
- Vavasour IM, Graf C, Kolind SH, et al. Advanced MRI measures reveal sex differences in the Normal Appearing and Diffusely Abnormal White Matter of Multiple Sclerosis Brain. International Society of Magnetic Resonance in Medicine 2020.
- Alexander DC, Hubbard PL, Hall MG, et al. Orientationally invariant indices of axon diameter and density from diffusion MRI. Neuroimage 2010; 52: 1374–1389.
- Daducci A, Canales-Rodríguez EJ, Zhang H, et al. Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. Neuroimage 2015; 105: 32–44.
- Zhang H, Schneider T, Wheeler-Kingshott CA, et al. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012; 61: 1000–1016.
- Descoteaux M, Angelino E, Fitzgibbons S, et al. Regularized, fast, and robust analytical Q-ball imaging. Magn Reson Med 2007; 58: 497–510.
- Schoonheim MM, Vigeveno RM, Lopes FCR, et al. Sex-specific extent and severity of white matter damage in multiple sclerosis: Implications for cognitive decline. Hum Brain Mapp 2014; 35: 2348–2358.
- Vermersch P, Oh J, Cascione M, et al. Teriflunomide vs injectable disease modifying therapies for relapsing forms of MS. Mult Scler Relat Disord 2020; 43: 102158.
- Kingsley PB. Introduction to diffusion tensor imaging mathematics: Part I. Tensors, rotations, and eigenvectors. Concepts Magn Reson Part A 2006; 28A: 101–122.
- Cohen JA, Barkhof F, Comi G, et al. Oral Fingolimod or Intramuscular Interferon for Relapsing Multiple Sclerosis. N Engl J Med 2010; 362: 402–415.
Figures
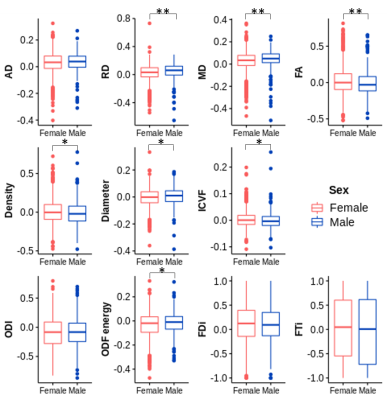
Figure 1.
Lesion-NAWM asymmetries compared between men and women. Diffusion tensor
imaging (top), high angular resolution compartment (middle), and diffusion orientation
analyses indicate differences in lesion-NAWM asymmetry between women and men. *p<0.05,
**p<0.01

Figure 2. Diffusion
metrics reflecting significant differences in microstructure pathology between
men and women. Calculation of diffusion measures took advantage of the A) average
diffusion b0. Measures showing significant sex-differences in microstructure
include B) radial diffusivity, C) mean diffusivity, and D) fractional
anisotropy diffusion tensor measures as well as E) axonal density, F) axonal
diameter, G) intracellular volume fraction and H) orientation distribution
function energy.
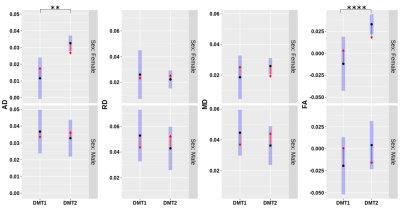
Figure 3. Diffusion
tensor imaging asymmetries are compared between DMT groups. Confidence intervals (blue) around the means
for axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD), and
fractional anisotropy (FA) normalized lesion values are compared in women (top)
and men (bottom). Comparison arrows (red) with no overlap indicate significance
of differences in estimated marginal means. AD and FA showed significant
differences between DMT1 (injectable) and DMT2 (oral) in women. *p0.05, **p<0.01,
***p<0.001, ****p<0.0001

Figure 4.
Lesion-NAWM AMICO-ActiveAx asymmetries are compared between DMT groups. Confidence intervals (blue) around the means
for density, diameter, and intracellular volume fraction (ICVF) normalized
lesion values are compared in women (top) and men (bottom). Comparison arrows
(red) with no overlap indicate significant differences in estimated marginal
means between injectable (DMT1) and oral (DMT2) therapies. In women, density
and ICVF show significant increases and diameter shows a significant decrease
in oral therapies. *p<0.05
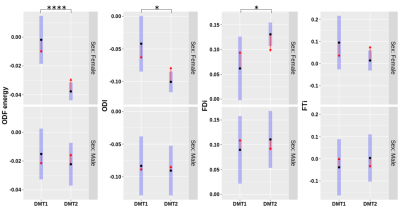
Figure 5.
Lesion-NAWM diffusion orientation model asymmetries are compared between DMT
groups. Confidence intervals (blue) around the means
for orientation distribution function (ODF) energy, orientation dispersion
index (ODI), and streamline density index (FDi), and streamline termination
index (FTi) normalized lesion values are compared in women (top) and men(bottom).
Comparison arrows (red) with no overlap indicate significant differences in
estimated marginal means between injectable (DMT1) and oral (DMT2) therapies. *p0.05,
**p<0.01, ***p<0.001, ****p<0.0001