2784
Application of MEGA-sLASER for detection of lipid composition in the human liver1Department of Radiology and Nuclear Medicine, NUTRIM School for Nutrition and Translational Research in Metabolism, Maastricht University Medical Center, Maastricht, Netherlands, 2Nutrition and Movement Sciences, NUTRIM School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands
Synopsis
1H-MRS has been widely used to measure total intrahepatic lipid content, but measuring lipid composition with specifically differentiating saturated, mono- and poly-unsaturated fatty acids in the liver, is challenging. At clinical field strength (3T), the allylic peak is overlapping with the alpha-carbonyl methylene resonance, which we recently addressed with a sophisticated fitting routine. However, this approach is difficult when shimming is suboptimal or in volunteers with low liver fat content (1-2%). Here, we evaluated the in vivo feasibility of J-difference editing, using MEGA-sLASER in the liver, to separate allylic from the alpha-carbonyl resonance for estimation of hepatic lipid composition.
Introduction
1H-MRS is widely used to measure total intrahepatic lipid (IHL) content1,2. In addition, from a physiological point of view, it is also important to determine hepatic lipid composition and specifically differentiate the saturated, mono- and poly-unsaturated fatty acid fraction (SFA, MUFA and PUFA respectively)3–5. In principle, the distinct detection of allylic (2.02 ppm) and diallylic (2.75 ppm) resonances allows estimating the MUFA and PUFA content, but at 3T, the allylic resonance is overlapping with alpha-carbonyl (2.24 ppm) peak. Recently, we tackled this problem by developing a sophisticated post-processing tool using appropriate prior knowledge and showed feasibility to measure hepatic lipid composition in humans3. Although this approach is accurate, it requires high quality spectra with optimal water suppression, and it is challenging when shimming is suboptimal, or liver fat is low (1-2%). Therefore, development of an alternative technique deserves our attention. In principle, sophisticated J-difference editing techniques6 (JDE, e.g., MEGA-sLASER) can be a potential alternative, which provides high specificity for MUFA and PUFA detection. MEGA-sLASER was successfully applied in skeletal muscle and adipose tissue to measure lipid composition7. However, it is not yet shown whether this technique is applicable to the liver where respiratory motion may lead to imperfect subtraction and therefore strong subtraction artifacts. Here, we evaluated the feasibility to detect and quantify lipid composition in the liver with the application of MEGA-sLASER8.Materials and methods
Experiments were performed on a 3T MR system (Achieva 3T-X Philips Healthcare, Best, Netherlands) with a 32-channel cardiac/torso coil (Philips Healthcare, Best, Netherlands). A MEGA-sLASER sequence was used and the editing efficiency of MEGA8 pulses (10 ms, Sinc-Gaussian pulse, bandwidth=180 Hz) was first tested in three different oil phantoms (peanut, rice and olive) with varying lipid composition, with the following parameters: TR/TE=4000/140 ms, NSA=32, Voxel=30x30x30 mm, datapoints=2048. In order to check in vivo feasibility, we applied MEGA-sLASER in four (m/f=2/2; Age=43±18 years; BMI=27.2±3.1 Kg/m2) healthy volunteers with varying IHL content, with the same experimental setup as applied in the oil phantoms, to measure hepatic lipid composition. The MEGA center ‘on’ frequency was kept at 5.31 ppm and ‘off’ resonance at 4.10 ppm (+76 and -76 Hz with respect to water) to ensure that water signal is equally affected in both acquisitions. Additionally, a narrow band (60 Hz) VAPOR9 water suppression was applied and a long TR (4000 ms) was chosen to let the subjects breath in the rhythm of the measurement (acquisition at end-expiration). A voxel (45x45x45 mm) was placed in the liver and 128 averages were acquired. In order to validate the protocol against our previously validated approach, hepatic lipid composition was also determined with STEAM in the same subjects as previously described3. Spectra were post-processed with a custom-written MATLAB script and target lipid resonances were integrated, and lipid composition was calculated as described before7:$$\%PUFA=CA\times\left(1.5\times\frac{\text{S}_{diallylic}}{\text{S}_{methyl}}\right)\times100$$
$$\%MUFA=CB\times\left[0.75\times\left(\frac{\text{S}_{allylic}}{\text{S}_{methyl}}\right)\right]\times100-\%PUFA$$
$$\%SFA=100-\%PUFA-\%MUFA$$
CA and CB are correction factors, which are empirically determined in oil phantoms, by dividing signal intensity of diallylic/CH3 and allylic/CH3 ratios (as determined by the editing approach at 3T) by the respective ratios as determined in high-resolution 1H-NMR (Bruker Avance III 700 MHz). The signal intensity of allylic peak, which required editing, was determined in the difference spectrum while the signal intensities for diallylic and methyl peaks were determined in the summed spectrum.
Results and discussion
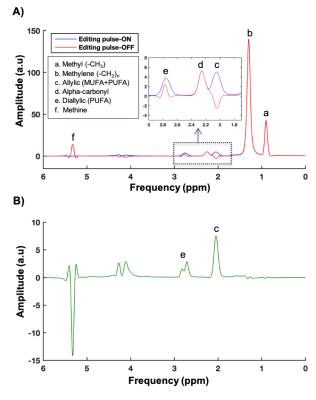
The MEGA-sLASER spectra acquired from peanut oil are depicted in figure 1. As expected, with target frequency for the MEGA pulses at 5.31 ppm, the coupled resonance at 2.02 ppm was refocused (zoomed part in figure 1A). Therefore, we were able to edit the target allylic peak (c) clearly in the subtracted spectrum, without contamination of the alpha-carbonyl resonance, as shown in figure 1B, showing good performance of the MEGA-sLASER. As expected, the correction factors were not much different among the three oils and the average correction factors were CA=2.58±0.19 and CB=7.87±0.51(mean ± standard deviation).A typical example of a JDE hepatic spectra is depicted in figure 2. The target allylic resonance is clearly visible as a single peak, not contaminated by other resonances, enabling the accurate estimation of MUFA and PUFA content in the liver, as shown in figure 2B. Although there is a small residual of lipid-CH2 signal (1.3 ppm) in the edited spectrum (figure 2B), it is not affecting the target resonance (c) showing the feasibility of using JDE approach to determine hepatic lipid composition also in the liver. Importantly, the measured hepatic lipid composition with the editing approach was in close agreement with the (previously validated) STEAM method3 (figure 3). As expected, SFA was not much differing (mean CV=6%) between two methods, however the MUFA and PUFA content especially on low liver fat subject was deviated in STEAM as compared to editing approach.
Conclusion
We demonstrated the applicability of MEGA-sLASER in the liver to detect hepatic lipid composition. This JDE approach can be used as an alternative technique to determine hepatic lipid composition in different populations. A TE of 140 ms is excellent for editing the allylic resonance, however, to improve the visibility of the diallylic resonance, acquisition of a spectrum with shorter TE could be added to the protocol in the future.Acknowledgements
This research was in part financed by the Ministry of Economic Affairs and Climate Policy by means of the PPP Allowance made available by the Top Sector Life Sciences & Health to stimulate public-private partnerships and by Unilever R&D Vlaardingen. LL was supported by a Veni grant from ZonMW (016.Veni.188.036). VBH is a recipient of an ERC starting grant (grant no. 759161 ‘MRS in diabetes’).References
1. Longo R, Pollesello P, Ricci C, et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging JMRI. 1995;5(3):281-285.
2. Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462-468.
3. Roumans KHM, Lindeboom L, Veeraiah P, et al. Hepatic saturated fatty acid fraction is associated with de novo lipogenesis and hepatic insulin resistance. Nat Commun. 2020;11(1):1891.
4. Luukkonen PK, Sädevirta S, Zhou Y, et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care. 2018;41(8):1732-1739.
5. Timmers S, de Vogel-van den Bosch J, de Wit N, et al. Differential effects of saturated versus unsaturated dietary fatty acids on weight gain and myocellular lipid profiles in mice. Nutr Diabetes. 2011;1(7):e11-e11.
6. Choi I-Y, Andronesi OC, Barker P, et al. Spectral editing in 1H magnetic resonance spectroscopy: Experts’ consensus recommendations. NMR Biomed. n/a(n/a):e4411.
7. Lindeboom L, Graaf RA de. Measurement of lipid composition in human skeletal muscle and adipose tissue with 1H-MRS homonuclear spectral editing. Magn Reson Med. 2018;79(2):619-627.
8. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266-272.
9. Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41(4):649-656.
Figures