2776
Free-Breathing, 3D Phase Sensitive Inversion Recovery MRI with Stack-of-Stars SGRE and Locally Low Rank Reconstruction
Yavuz Muslu1,2, Ty A. Cashen3, Sagar Mandava4, and Scott B. Reeder1,2,5,6,7
1Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Global MR Applications and Workflow, GE Healthcare, Madison, WI, United States, 4Global MR Applications and Workflow, GE Healthcare, Atlanta, GA, United States, 5Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 6Department of Medicine, University of Wisconsin-Madison, Madison, WI, United States, 7Department of Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
1Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Global MR Applications and Workflow, GE Healthcare, Madison, WI, United States, 4Global MR Applications and Workflow, GE Healthcare, Atlanta, GA, United States, 5Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 6Department of Medicine, University of Wisconsin-Madison, Madison, WI, United States, 7Department of Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Gadoxetic acid (GA)-enhanced MRI is an important tool for the detection of liver metastases. Through GA dose optimization and novel pulse sequence strategies, small lesions are often resolved using only high resolution GA-enhanced hepatobiliary phase T1w-MRI. However, such small lesions are often occult in T2w, diffusion weighted imaging (DWI) and dynamic contrast enhanced (DCE) T1w-MRI, which hinders the ability of MRI to characterize these lesions. In this work, we propose the development of a phase sensitive inversion recovery (PSIR) T1w-MRI in combination with 3D radial stack-of-stars imaging for detection and characterization of small focal liver lesions.
Introduction
Gadoxetic acid (GA)-enhanced hepatobiliary phase (HBP) T1 weighted (T1w)-MRI is an established and effective tool for detection of small (<1cm) liver lesions1,2. When combined with T2w, DWI or DCE-T1w-MRI, HBP-T1w imaging is capable of accurate detection and characterization of small lesions such as metastases. Unfortunately, the performance of T1w-MRI in the HBP often exceeds that of other methods, where small lesions are occult on all other sequences except T1w-HBP imaging3-7. As a result, such lesions are interpreted as “indeterminate”, creating considerable uncertainty regarding the management of these lesions, including surgical planning for curative hepatectomy and/or chemotherapy.In IR-based T1w-MRI, an inversion pulse (180o) is applied prior to k-space readout (e.g. SGRE, SSFP) to increase the T1 contrast of the images. Two main reconstruction schemes for such sequences include magnitude and phase sensitive (PS) reconstructions. The performance of magnitude reconstruction relies on the optimal inversion time (TI) selection to maximize the contrast and can suffer from ambiguity since tissues with both short and long T1 relaxation appear bright on magnitude images. PS reconstruction avoids this ambiguity by capturing the true phase of the spins, furthermore, improves the contrast by utilizing the full dynamic range of the longitudinal magnetization8.
Radial stack-of-stars k-space trajectory, in combination with golden-angle projection order, offers important advantages for abdominal MRI, including motion robustness during free-breathing acquisitions, incoherent undersampling artifacts that can be leveraged in a compressed sensing reconstruction, and self-navigation capabilities9-11. Furthermore, frequent sampling of center of the k-space allows for more flexible acquisition and reconstruction schemes especially when combined with magnetization preparation.
In this work, we propose the development of a phase sensitive inversion recovery (PSIR) T1w-MRI in combination with 3D radial stack-of-stars imaging for detection and characterization of small focal liver lesions.
Methods
MRI Acquisition: An adiabatic, spatially non-selective inversion pulse is used to introduce T1 weighting, followed by the acquisition of a series of radial stacks of kz-encodes, rotated by the golden angle increments10. The number of radial stacks and kz-encoding lines is determined by the desired temporal resolution and T1 relaxation of the tissue of interest. After the acquisition of radial stacks, further longitudinal recovery is allowed by creating a “dead time” (TD). Dual-echo imaging with Dixon water-fat separation is used for fat suppression. Magnetization preparation and acquisition scheme is illustrated in Figure 1.Phase Sensitive Inversion Recovery: Dual-echo (in-phase and out-of-phase) radial stack-of-stars (SOS) images were acquired in one volunteer (male, age=33) on a 3T MR scanner (GE Healthcare Discovery MR750, Waukesha, WI) using a 32-channel phased array torso coil. Imaging parameters were as follows: FOV=540x540mm2, resolution=1.1x1.1 mm2, slice thickness=3.0mm, flip angle=8o, number of slices=58, partial Fourier readout fraction=70%, TR/TE1/TE2=5/1.4/2.7ms, Bandwidth=±167kHz. 12 radial stacks were acquired after each inversion pulse. Last radial stack, before the dead time, was acquired as a phase reference with smaller flip angle (4o) to minimize T1 weighting8. Dead time was set to 500ms12, leading to total acquisition time of 8.5 minutes.
Image Reconstruction: Locally low rank (LLR) reconstruction is applied to basis coefficients of separate echo images13. Low rank constraint in time dimension is applied through temporal basis transformation, where basis functions for T1 relaxation are calculated using Bloch equation simulations, covering a wide range of T1 (200-3000ms) values, B1 inhomogeneities (0.5-1.5) and inversion efficiencies (0.5-1). Once the dual echo images reconstructed, last time frame is used as the phase reference for the phase sensitive processing8. GE Orchestra Toolbox is used to generate water and fat images from PSIR images separately for each TI frame.
Results
Figure 2 shows LLR+magnitude reconstructed IR-T1w out-of-phase and in-phase images reconstructed at different effective TIs. Note the ambiguity of image contrast at early and late time frames. Figure 3 shows the water images at different effective TIs after phase sensitive processing and 2-point Dixon water/fat separation, capturing the true T1 recovery behavior of the tissues. Phase sensitive image contrasts are consistent with the T1 relaxations of different tissue types, i.e. at 3T without any contrast enhancement, subcutaneous fat shows the quickest recovery, followed by liver parenchyma, blood and water in the stomach.Discussion
In this work, we presented an image acquisition and reconstruction scheme for free-breathing, 3D PSIR-MRI in the liver. Phase sensitive image reconstruction captures the true phase of the spins and takes full advantage of dynamic range of T1-weighting in an IR-prepared image acquisition. Stack-of-Stars sampling minimizes the artifacts due to respiration. Future work includes further reductions in acquisition duration, improvements in fat suppression and volunteer/patient studies.Acknowledgements
We wish to acknowledge investigator-initiated research support from Bayer, UW Institute for Clinical and Translational Research, and the Clinical and Translational Science Award of the NCATS/NIH (UL1TR002373). Further, we wish to acknowledge GE Healthcare who provides research support to the University of Wisconsin. Finally, Dr. Reeder is a Romnes Faculty Fellow, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.References
- Bashir MR, Husarik DB, Ziemlewicz TJ, Gupta RT, Boll DT, Merkle EM. Liver MRI in the hepatocyte phase with gadolinium-EOB-DTPA: does increasing the flip angle improve conspicuity and detection rate of hypointense lesions? Journal of magnetic resonance imaging: JMRI. 2012;35(3):611-6.
- Vilgrain V, Esvan M, Ronot M, Caumont-Prim A, Aubé C, Chatellier G. A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases. European Radiology. 2016;26(12):4595-615.
- Frydrychowicz A, Nagle SK, D'Souza SL, Vigen KK, Reeder SB. Optimized high-resolution contrast-enhanced hepatobiliary imaging at 3 tesla: a cross-over comparison of gadobenate dimeglumine and gadoxetic acid. Journal of magnetic resonance imaging: JMRI. 2011;34(3):585-94
- Motosugi U, Bannas P, Hernando D, Rahimi MS, Holmes JH, Reeder SB. Intraindividual Crossover Comparison of Gadoxetic Acid Dose for Liver MRI in Normal Volunteers. Magnetic Resonance in Medical Sciences. 2016;15(1):60-72.
- Nagle SK, Busse RF, Brau AC, Brittain JH, Frydrychowicz A, Iwadate Y, Reeder SB. High resolution navigated three-dimensional T₁-weighted hepatobiliary MRI using gadoxetic acid optimized for 1.5 Tesla. Journal of magnetic resonance imaging: JMRI. 2012;36(4):890-9.
- Muehler M, Cashen T, Wang K, Ersoz A, Bayram E, Reeder SB, editors. 3D Stack-of-Stars Radial Imaging for Motion-Robust Free-Breathing Hepatobiliary Phase Imaging. ISMRM; 2019 13 May 2019.
- Bannas P, Motosugi U, Hernando D, Rahimi MS, Holmes JH, Reeder SB. Combined gadoxetic acid and gadofosveset enhanced liver MRI: A feasibility and parameter optimization study. Magnetic Resonance in Medicine. 2016;75(1):318-28.
- Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-Sensitive Inversion Recovery for Detecting Myocardial Infarction Using Gadolinium-Delayed Hyperenhancement. Magnetic Resonance in Medicine. 2002; 47: 372-383.
- Kecskemeti, S., Johnson, K., Wu, Y., Mistretta, C., Turski, P. and Wieben, O. (2012), High resolution three‐dimensional cine phase contrast MRI of small intracranial aneurysms using a stack of stars k‐space trajectory. J. Magn. Reson. Imaging, 35: 518-527.
- Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med 2014;72(3):707-717.
- Feng, L., Axel, L., Chandarana, H., Block, K.T., Sodickson, D.K. and Otazo, R. (2016), XD‐GRASP: Golden‐angle radial MRI with reconstruction of extra motion‐state dimensions using compressed sensing. Magn. Reson. Med., 75: 775-788
- Kecskemeti, S., Samsonov, A., Hurley, S.A., Dean, D.C., Field, A. and Alexander, A.L. Mpnrage: A technique to simultaneously acquire hundreds of differently contrasted mprage images with applications to quantitative t1 mapping. Magn Reson Med. 2016; 75(3):1040–1053.
- Tamir, J.I., Uecker, M., Chen, W., Lai, P., Alley, M.T., Vasanawala, S.S. and Lustig, M. T2 shuffling: Sharp, multicontrast, volumetric fast spin‐echo imaging. 2017 Magn. Reson. Med., 77: 180-195
Figures
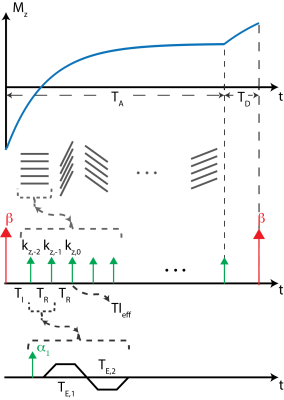
Figure 1: Pulse sequence diagram for the proposed radial stack of stars T1w-PSIR acquisition. After each IR pulse, stacks of kz-encodes are acquired. TA represents “active time” during which the acquisition continues. “Dead time”, TD, is time allowed for free magnetization recovery. TIeff represents effective TI of a time frame, marked by the acquisition of central kz-encode. Dual echo acquisition is achieved by bipolar readout gradients for each kz-encode.
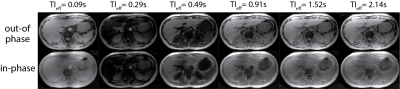
Figure 2: IR based T1w-MRI can impart significant T1 weighting into images acquired at different inversion times. However, magnitude reconstructed images come with susceptibility to TI selection. Shown are the LLR+magnitude reconstruction of out-of-phase, in-phase images at different TI times. LLR reconstruction in combination with the proposed acquisition method provided good image quality. Susceptibility to selection of TI is observed in short and long T1 species, which are bright in both early and late time frames.
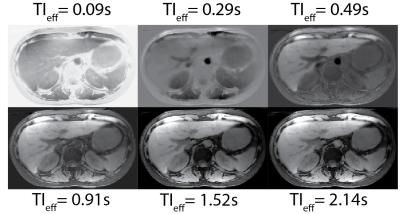
Figure 3: Phase sensitive processing can be applied to IR-SoS T1w-MRI acquisitions to unwrap the phase and take full advantage of the dynamic range of T1 recovery. Shown are the PSIR water-only images reconstructed at different effective TI times. True T1 recovery behavior after the IR preparation is captured with phase sensitive processing, removing the susceptibility to TI selection. 2-point Dixon water/fat separation successfully suppressed fat signal, resulting in better image contrast in the liver.