2770
Hepatobiliary phase hypointensity improves the diagnostic performance of version 2018 LI⁃RADS on hepatocellular carcinoma1Department of Radiology,Affiliated Hospital of Guilin Medical University, Guilin, China, 2MR Research, GE Healthcare, Beijing, China
Synopsis
Hypointensity in the hepatobiliary phase is an important ancillary feature in the diagnosis of liver malignancies in the version 2018 of LI-RADS. In this study, Hypointensity in the hepatobiliary phase was used as the major features to explore the effect of hepatobiliary hypointensity on the diagnosis performance of LI-RADS in small hepatocellular carcinoma (sHCC). Our results indicated that the sensitivity of diagnosing sHCC can be significantly improved without affecting the specificity of diagnosing sHCC when Hypointensity in the hepatobiliary phase was used as the major features for classification of LR-3,LR-4.
Introduction
Hepatocellular carcinoma is one of the most common malignant tumors in China, and the accuracy of its clinical diagnosis is directly related to the clinical treatment decision and prognosis of patients, especially sHCC . The 5-year overall survival rate after sHCC surgical resection or radiofrequency ablation is 61.8% to 71.9%[1].Therefore, early screening and detection are the only effective treatment for high-risk HCC patients. Hypointensity in the hepatobiliary phase is an important ancillary features for the diagnosis of liver malignancies in the version 2018 of Liver Imaging Report and Data System (LI-RADS)[2].The aim of this study was to investigate the effect of Gd‑EOB‑DTPA enhanced hypointensity in the hepatobiliary phase on the diagnostic efficacy of the version 2018 of LI-RADS in sHCC.Methods
This retrospective study was performed on 157 high-risk HCC patients who received Gd‑EOB‑DTPA enhanced MRI examination in our hospital from January 2014 to April 2020. Patients were scanned on 3.0T MRI with a 16-channel phased array body coil, and Gd‑EOB‑DTPA contrast agent(Bayer Healthcare, Germany). After pre-scanning, 0.025mmol/kg Gd‑EOB‑DTPA contrast agent was intravenously injected with an injection flow rate of 1 ml/s followed by 20 ml normal saline. After injection of Gd‑EOB‑DTPA contrast agent, images at arterial phase, portal venous phase, delayed phase, transitional phase , and hepatobiliary phases were acquired. The two radiologists double-blindly review and analyze patient images, recorded the major and ancillary features for each observation, and classified disease stages according to the version 2018 of LI-RADS diagnostic table. The inter-reader reliability of LI-RADS categories and major and ancillary features of observations between two radiologists were calculated by Kappa test. Continuous variables were presented as means and standard deviations (SD). The categorical variables are presented as counts and percentages. The diagnostic performance of LI-RADS feature and classification was presented by sensitivity, specificity, accuracy, positive and negative predictive values.Results
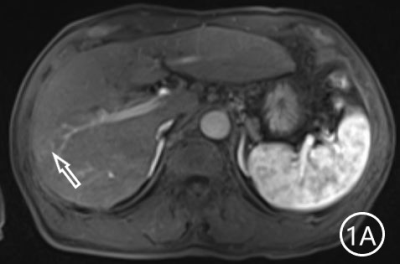
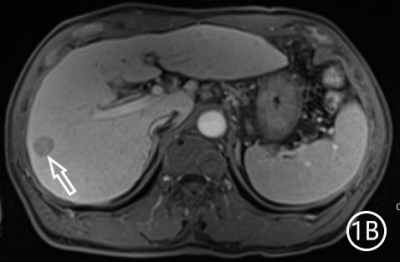
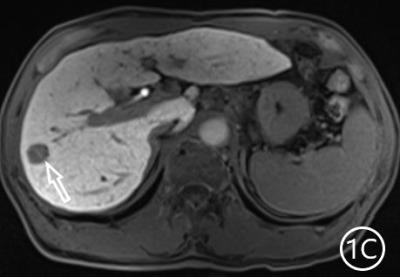
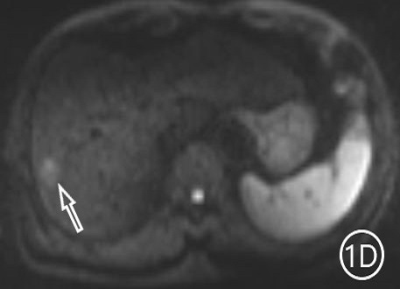
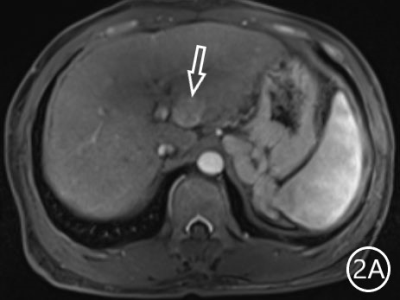
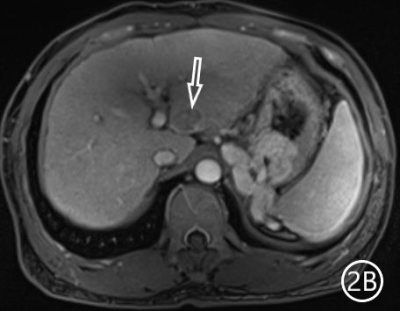
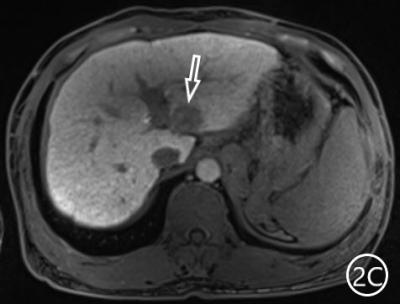
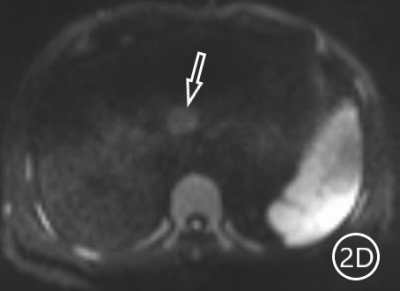
The two radiologists recorded the major features for each observation, hypointensity in the hepatobiliary phase, classification based on LI-RADS. A Kappa test was used to check consistency (Kappa values of more than 0.80 means high consistency). The sensitivity, specificity and accuracy of the two radiologists in diagnosing sHCC based on hypointensity in the hepatobiliary phase were 98.66%, 72.22% and 95.81%, respectively, and the positive predictive value was 96.71%. The sensitivity, specificity and accuracy of the two radiologists in diagnosing sHCC as LR-4/LR-5 were 93.29%, 66.67% and 90.42%, and the positive predictive value was 95.86%. (Table 1)There were 11 observations in LR-3 show as hypointensity in the hepatobiliary phase, and 10 observations update to LR-4 because of the hypointensity in the hepatobiliary phase.The 10 observations contained 9 sHCC and 1 HGDN (Fig 1、2).Another LR-3 observation maintained the original classification.The sensitivity, specificity and accuracy of the two radiologists in diagnosing sHCC as LR-4/LR-5 after adjusting LR-3 to LR-4 based on hypointensity in the hepatobiliary phase were 99.33%, 61.11% and 95.21%, and the positive predictive value was 95.48%, when the hypointensity in the hepatobiliary phase was taken as the major features to adjust the classification of LR-3 and LR-4(Table 2).Discussion
In this study, hypointensity in the hepatobiliary phase demonstrated high sensitivity, accuracy and positive predictive value to diagnose HCC in high‐risk patients on Gd‐EOB‐DTPA enhanced MR images, but the specificity of hypointensity in the hepatobiliary phase was relatively fair. The mainly reason may be only 3 cases respectively with low-grade dysplastic nodule (LGDN) and high-grade dysplastic nodules (HGDN) were included in this study. The HGDN originally classified as LR-3 was regarded as LR-4 in our study based on the hypointensity in the hepatobiliary phase. Therefore, the specificity of LR-4/LR-5 in diagnosing sHCC decreased. HGDN was characterized by arterial enhancement and hypointensity in the hepatobiliary phase mainly due to the formation of unpaired tumor arteries, hepatic sinusoidal capillarization[3] and decreased OATP expression[4], leading to imaging manifestations of HGND similar to those of early hepatocellular carcinoma, and consequently the diagnostic efficacy of hypointensity in the hepatobiliary phase declined. As a precancerous lesion of HCC, the prognosis of HGDN was significantly higher than that of early and advanced hepatocellular carcinoma [5]. Therefore, it is necessary to further explore a new feature or develop new classifications for the diagnose HCC precancerous lesions so as to improve the efficacy of hypointensity in the hepatobiliary phase in the diagnosis of HCC.Conclusion
Diagnostic performance of version 2018 LI⁃RADS demonstrated high sensitivity, specificity and accuracy of diagnosing sHCC in high⁃risk patients based on Gd⁃EOB⁃DTPA enhanced MRI. Hypointensity in the hepatobiliary phase can improve the diagnostic performance of LI-RADS for sHCC.Acknowledgements
This work was supported by the Guangxi Key Laboratory of Molecular Medicine for Liver Injury and Repair (grant numbers: GXLIRMMKL-K202010) and Major Special Project of Guilin Scientific Research and technology Development Plan (grant numbers: 20190202-2).References
[1]. Xu Q, Kobayashi S, Ye X. et al. Comparison of Hepatic Resection and Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Meta-Analysis of 16,103 Patients. Sci Rep 4, 7252 (2014). https://doi.org/10.1038/srep07252
[2]. American College of Radiology.CT/MR Liver Imaging Reporting and Data System version2018[EB/OL].https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018.
[3]. Park HJ, Choi BI, Lee ES, et al. How to differentiate borderline hepatic nodules in hepatocarcinogenesis: emphasis on imaging diagnosis [J]. Liver Cancer, 2017, 6(3): 189-203.
[4]. Kitao A, Matsui O, Yoneda N, et al. The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging [J]. Eur Radiol,2011, 21(10): 2056-2066.
[5].《中华肝脏病杂志》编辑委员会. 中华医学会肝病学分会肝癌学组.肝细胞癌癌前病变的诊断和治疗多学科专家共识( 2020版) [J]. 临床肝胆病杂志, 2020, 36(3): 514-518.
Figures