2762
Diagnostic value of radiomics analysis based on multimodal MRI for advanced liver fibrosis in patients with hepatitis B1Nantong University Affiliated Nantong Third People's Hospital, Nantong, China, 2Philips Healthcare Shanghai, China, Shanghai, China
Synopsis
The purpose of this study was to develop and validate an optimal radiomics-based model for diagnosis of advanced liver fibrosis. Firstly, multimodal MRI was performed before and 20 minutes after Gd-EOB-DTPA administration. Secondly, the clinical diagnosis model, fusion radiomics signature and radiomics nomogram model were established in the training cohort. Finally, the diagnostic value of three models was confirmed in the validation cohort.
Introduction
Accurate assessment of hepatic fibrosis stage and timely treatment can effectively control the progression, which is of great significance in improving the prognosis of patients[1, 2]. Among the variety of quantitative techniques for estimating liver fibrosis, radiomics as an artificial intelligence strategy has become a research hotspot[3,4]. The purpose of this study was to develop and validate an optimal radiomics-based model for diagnosis of advanced liver fibrosis.Purpose
To develop and validate an optimal radiomics-based model for diagnosis of advanced liver fibrosis.Methods
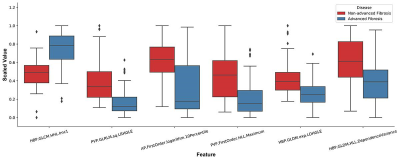
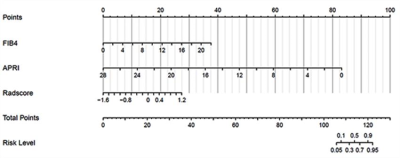
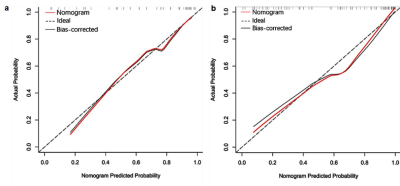
143 patients with hepatitis B fibrosis were randomly divided into training cohort (95 cases) and validation cohort (48) in a 2:1 ratio. Clinical data of 143 patients were analyzed retrospectively. In the training cohort, independent risk factors for advanced liver fibrosis were analyzed and the related clinical diagnosis model was established with Logistic regression, the fusion radiomics signature model of MRI was established with linear support vector classification (SVC) using six radiomics features (Figure 1), and a radiomics nomogram model integrated the fusion radiomics signature and independent risk factors(Figure 2), then the prediction value of the nomogram model for advanced liver fibrosis was assessed with calibration curve. The diagnostic value of three models in training cohort was assessed by ROC analysis, and then confirmed in the validation cohort.Results
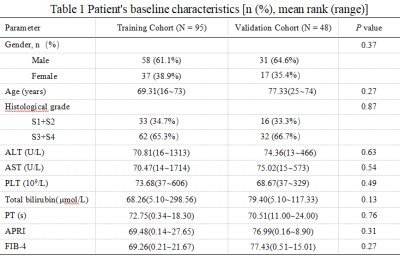
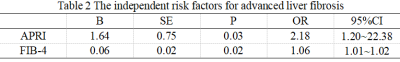
Clinical CharacteristicsThere were no significant differences in clinical factors between the two cohorts (P>0.05) (Table 1). APRI and FIB-4 were the independent risk factors for advanced liver fibrosis (P<0.05) in the training cohort (Table 2).
The diagnostic value of three models in training cohort
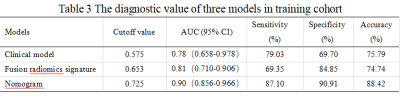
Nomogram presented good prediction probability for advanced liver fibrosis (Figure 3a). The AUC of nomogram model was higher than that of the clinical model and fusion radiomics signature model (P<0.05), and the nomogram model had better diagnostic value (Table 3).
The diagnostic value of three models was confirmed in the validation cohort
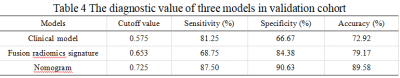
Nomogram presented good agreement in the prediction probability for advanced liver fibrosis between the training and validation cohorts (Figure 3a,b). The cutoff value of three models gained in the training cohort were applied to the validation cohort, and the results showed the sensitivity, specificity and accuracy of three models were similar to the training cohort (Table 4).
Discussion
Our study aimed to perform radiomics analysis based on multimodal MRI in identifying advanced liver fibrosis. S3 and S4 stage disease were grouped together into the advanced liver fibrosis group because the S3-S4 fibrosis was more serious. Our results demonstrated that the fusion radiomics signature obtained from AP, PVP and HBP images and the fusion radiomics signature diagnosis model could diagnose patients with stage S3-S4 disease with high sensitivity. Moreover, a combined nomogram model that combined significant clinical factors with the fusion radiomics signature was developed and validated, and it exhibited better performance in diagnosing hepatic fibrosis S3-S4 than the fusion radiomics signature or clinical model alone.Conclusions
In conclusion, radiomics analysis based on multimodal MRI has good diagnostic value for advanced liver fibrosis and the nomogram model integrating clinical data with radiomics analysis can enhance the diagnostic value.Acknowledgements
NoneReferences
1. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, (4), 1560-1599.
2. Schuppan D, Kim YO. Evolving therapies for liver fibrosis. The Journal of clinical investigation 2013, 123, (5), 1887-901.
3. Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, (2), 563-77.
4. Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nature reviews. Clinical oncology 2017, 14, (12), 749-762.
Figures