2757
A quantitative perfusion value is a good discriminator of liver fibrosis level in patients with chronic hepatitis B1Guangdong Hospital of Traditional Chinese Medicine, Zhuhai, China, 2MR Research, GE Healthcare, Beijing, China 100176, Beijing, China
Synopsis
IVIM is reckoned as a valuable tool for non-invasive detection and evaluation of liver fibrosis staging. However, only more small b values used in one scan improve the diagnosis of liver fibrosis. In clinics, the different fibrosis stages overlap in the same inflammation level. However, both physiological expressions were different. To distinguish fibrosis from inflammation on images may help clinical diagnosis and provide more effective treatments for patients. Our study demonstrated that Dfast indeed had excellent discrimination ability of early liver fibrosis S1 and S2 in CHB patients with the same inflammation grade.
Introduction
IIntravoxel incoherent motion (IVIM) is considered to be an effective tool for non-invasive detection and evaluation of liver fibrosis staging[1-3] [1-3]. Cohen et al. mentioned at least two low b values should be utilized to assure the accuracy of liver IVIM studies [4]. Three or more low b values (0 < b < 50 s/mm2) achieve higher diagnostic performance in detecting fibrosis [1]; however, more b values used in one examination increase the scanning time. In addition, a laboratory test of hepatic inflammation grade and fibrosis stage on a liver tissue sample retrieved via ultrasound-guided liver biopsy is commonly used as the standard. However, fibrosis stages overlap in the same inflammation level in clinic diagnosis criteria. The nonlinear relation of fibrosis stage and inflammation may disturb interactions and cause confounding effect when diagnosis. Till now, no relationship between different fibrosis stage in the same patients with the same inflammation grade has been explored. To trade off time and accuracy of image data, 7 lower b values (b<200 s/mm2) was used in our study. The purpose of this study was to investigate if IVIM-based parameters can differentiate fibrosis stage S1 from S2 among the patients with the same inflammation level (G2).Materials and Methods
10 patients with confirmed chronic hepatitis B in accompany with liver fibrosis stage (S1-S2) and inflammation grade (G2) were recruited. Three ROIs of 80-100 mm2 were placed on S6 of right liver where liver biopsy was also performed while avoiding large vessels. Perfusion fraction (f), pseudo-diffusion coefficient (Dfast), diffusion coefficient (Dslow) of IVIM diffusion imaging with 13 b values (0, 25, 50, 75, 100, 150, 200, 300, 400, 500, 600, 800,0 s/mm2) using two-segment mono-exponential model and bi-exponential model as well as apparent diffusion coefficient (ADC) using single exponential model were measured and analyzed by SPSS 21.0 with independent Samples test or Wilcoxon test. Independent Samples test or Mann-Whitney U test was used to observe whether the measured variables (ADC, dslow, dfast, f) in S1 group and S2 group were different.Results:
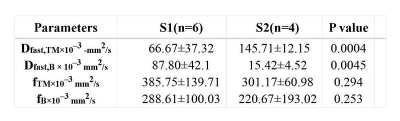
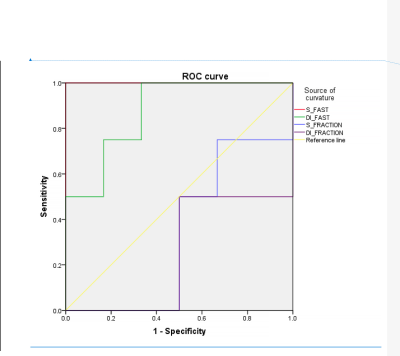
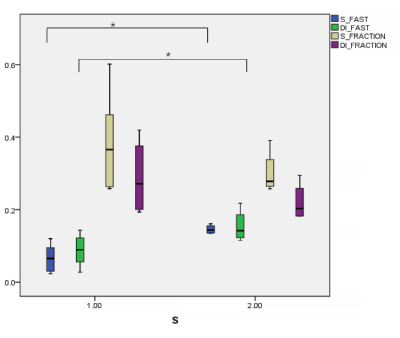
Significantly differences of Dfast,TM and fTM (p = 0.004 and 0.294) computed by the two-segment mono-exponential model as well as Dfast,B and fB (p = 0.045 and 0.253) computed by the bi-exponential model between S1 and S2 groups were found. The diagnostic performance of Dfast, TM (AUC= 1.00, p = 0.011) and Dfast, B (AUC= 0.875, p = 0.055) in distinguish S1 from S2 were good while that of fTM (AUC= 0.333, p=0.394) and fB (AUC= 0.250, p=0.201) were poor.Discussion
Our study found that the discrimination ability of Dfast,TM based on the two-segment mono-exponential model on S1 and S2 patients with the same level (G2) was better than Dfast,B, but all were statistically meaningful, in consistency with Hu et al[5]. In accordance to the setting in Hu et al[5], our study showed the number of low b values definitely affect Dfast and repeatability [4]. Dfast represents the pseudo diffusion effect caused by microcirculation perfusion in tissues. The lower the b value, and the closer to the real micro-perfusion the Dfast is. With the increase of hepatic fibrosis, the microcirculation perfusion volume gradually incresases; therefore, Dfast can be used for the staging of early liver fibrosis [3, 6, 7]. Our study demonstrated the difference of Dfast, TM (P < 0.004) was more obvious than Dfast, B (P < 0.045). However, it is difficult to rule out the contingency and prove that Dfast, TM is more advantageous than Dfast, B in the grading liver fibrosis. In addition, few studies indicated perfusion fraction (f) has a diagnostic value for fibrosis staging [8]. In our study, no difference of f between S1 and S2 groups was found in two-segment mono-exponential model or bi-exponential model. This result may be related to the insufficient number of patients or more low b values were used in our study. Therefore, it is necessary to increase sample size to assure the current results in the future.Conclusion
Dfast analyzed based on diffusion weighted images with multi-b values can be used to grade liver fibrosis, especially in differentiating different level of liver fibrosis in the same patients with the same inflammation level.Acknowledgements
No acknowledgement found.References
1. Ye, Z., et al., Value of intravoxel incoherent motion in detecting and staging liver fibrosis: A meta-analysis. World Journal of Gastroenterology, 2020. 26(23): p. 3304-3317. 2. Fu, F., et al., Non-invasive assessment of hepatic fibrosis: comparison of MR elastography to transient elastography and intravoxel incoherent motion diffusion-weighted MRI. Abdominal Radiology, 2020. 45(1): p. 73-82. 3. Jeong, et al., Comparison of monoexponential, intravoxel incoherent motion diffusion-weighted imaging and diffusion kurtosis imaging for assessment of hepatic fibrosis. Acta Radiologica, 2019. 4. Cohen, A.D., et al., The effect of low b-values on the intravoxel incoherent motion derived pseudodiffusion parameter in liver. Magnetic Resonance in Medicine, 2015. 73(1): p. 306-311. 5. Hu, F., et al., Liver fibrosis: in vivo evaluation using intravoxel incoherent motion-derived histogram metrics with histopathologic findings at 3.0 T. Abdominal Radiology, 2017. 42(12): p. 2855-2863. 6. Sandrasegaran, K., et al., Does intravoxel incoherent motion reliably stage hepatic fibrosis, steatosis, and inflammation? Abdom Radiol (NY), 2018. 43(3): p. 600-606. 7. Seo, N., et al., Liver fibrosis: stretched exponential model outperforms mono-exponential and bi-exponential models of diffusion-weighted MRI. Eur Radiol, 2018. 28(7): p. 2812-2822. 8. Chung, S.R., et al., Intravoxel incoherent motion MRI for liver fibrosis assessment: a pilot study. Acta Radiologica, 2015. 56(12): p. 1428.
Figures