2751
Measurement of Pulmonary Perfusion under Expiratory and Inspiratory Breathing Conditions using PCASL-bSSFP Imaging at 1.5 Tesla1Section on Experimental Radiology, University of Tübingen, Tübingen, Germany, 2Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 3Institute of Signal Processing and System Theory, University of Stuttgart, Stuttgart, Germany, 4Department of Diagnostic and Interventional Radiology, University of Tübingen, Tübingen, Germany, 5Department of Biomedical Magnetic Resonance, University of Tübingen, Tübingen, Germany
Synopsis
Pseudo-continuous-arterial-spin-labeling (PCASL) has been successfully applied in the lung providing high quality perfusion images. The pulmonary blood flow and the respiratory system interact closely: the intrathoracic pressure has impact on the venous return. Therefore, in this work, we evaluate the effects of intrathoracic pressure on lung perfusion by using PCASL imaging in end-expiratory and end-inspiratory breath-hold. PCASL imaging is able to detect changes of parenchymal lung perfusion caused by alterations of the intrathoracic pressure. Perfusion signal measured under end-inspiratory condition were noticeably reduced as compared to end-expiratory breath-hold. This correlated significantly with measured blood flow volume through the pulmonary trunk.
Introduction and Purpose
Measurement of pulmonary perfusion and visualization of its spatial distribution can be of clinical significance in diseases affecting the pulmonary vessels, the pulmonary interstitium or in bronchial carcinoma.1-3 Although the lungs receive the entire cardiac output, perfusion imaging using MRI is still a challenging task due to the low proton density, the breathing movements and the high pulsatility of the pulmonary circulation. Recently, an ECG-triggered pseudo-continuous ASL (PCASL) approach using balanced steady-state free-precession (SSFP) imaging was applied for perfusion measurements of the lung and provides high image quality and reliable quantitative perfusion maps even under free-breathing conditions.4 However, the pulmonary blood flow and the respiratory system influence each other: pulmonary vascular resistance, for example, is correlated to the lung volume and the intrathoracic pressure has impact on the venous return.5 In this work, we are aiming to evaluate the effects of intrathoracic pressure on lung perfusion by using PCASL-bSSFP imaging in end-expiratory and end-inspiratory breath-hold.Methods
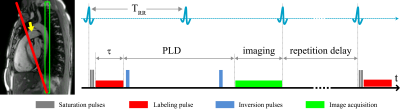
Four healthy volunteers (age 28.8±3.6 years, male) were examined on a 1.5T MR scanner (Avantofit, Siemens Healthcare, Erlangen, Germany). Three respiration schemes were performed: 1) expiratory breath-hold; 2) inspiratory breath-hold; 3) and free-breathing. For each respiration scheme, the lung perfusion and the blood flow of the pulmonary trunk (TP) were measured. The perfusion measurements were performed using the ECG-triggered PCASL-bSSFP sequence by labeling the pulmonary trunk during the systolic cardiac period (Figure 1). Labeling parameters were: duration, 400 ms; post-labeling delay, 1000 ms; flip angle, 25°; repetition delay, 3 sec. Coronal single-slice images were acquired in diastole of the next cardiac cycle using following parameters: TR, 2.1 ms; TE, 0.9 ms; flip angle, 70°; slice thickness, 20 mm; in-plane resolution, 2.5×2.5 mm2; matrix size, 144×192; readout bandwidth, 1260 Hz/pixel. In breath-hold examinations, one label-control image pair was acquired and twelve label-control image pairs were measured under free-breathing condition. A proton-density weighted bSSFP image was also acquired at the start of each scan. The scan time of breath-hold and free-breathing measurements was approx. 13 sec and 2 min, respectively. Blood flow measurements of TP was performed using standard phase-contrast imaging. For expiratory and inspiratory breath-hold examinations prospective triggering was used and retrospective gating was applied in free-breathing flow measurements. PCASL images were registered prior to further evaluation. Image registration were conducted by LAP6,7 using an in-house developed MATLAB (The MathWorks, Natick, MA) script. Perfusion signal was quantified in manually drawn regions of interest in perfusion-weighted (Control-Label) images. ROIs were carefully placed in the peripheral parenchyma of the right and left lung to avoid contribution of macroscopic vessels. The TP blood flow was quantified using Argus software on the scanner (Siemens Healthcare, Erlangen, Germany). The relationship between parenchymal perfusion and TP blood flow volume was calculated using Pearson’s correlation coefficient.Results
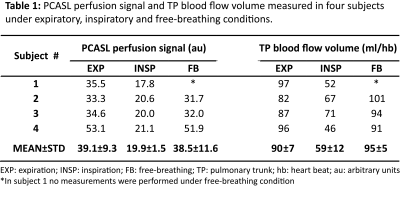
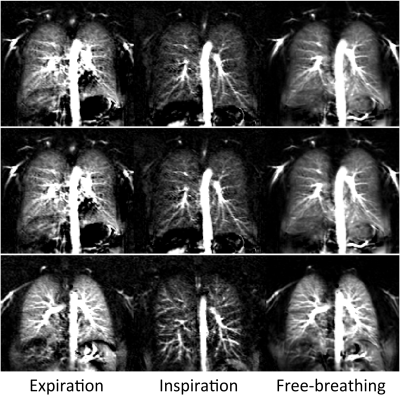
PCASL perfusion measurements of the lung and TP blood flow measurements of healthy volunteers were successfully performed under all three respiratory conditions (Table 1). Perfusion signal values (mean±std) averaged over all subjects were 39.1±9.3 and 19.9±1.4 in expiratory and inspiratory breathing conditions, respectively. Corresponding mean values for TP blood flow volume were 90±7 ml/hb and 59±12 ml/hb. The results show a significant reduction of parenchymal perfusion signal as well as TP blood flow volume measured in inspiration compared to expiration. The relative changes in perfusion signal and TP blood flow volume for expiratory and inspiratory conditions are highly correlated (Pearson, 0.93). In contrast, the perfusion signal measured in free breathing is almost identical to the values in expiration. Figure 2 shows PCASL-bSSFP perfusion-weighted images of three healthy volunteers measured under expiratory, inspiratory and free-breathing conditions. Perfusion images acquired in expiratory breath-hold and free-breathing show high signal in lung parenchyma. Both measurements reveal no significant difference in perfusion signal. Free-breathing perfusion images show higher SNR due to averaging of multiple measurements.Discussion and Conclusion
The results of this preliminary study demonstrate that PCASL-bSSFP sequence is able to detect changes of parenchymal lung perfusion caused by changes of the intrathoracic pressure. Perfusion signal measured under end-inspiratory condition was noticeably reduced as compared to end-expiratory breath-hold. This correlated significantly with the measured blood flow volume through the pulmonary trunk. In contrast, the parenchymal perfusion signal as well as TP blood flow volume measured under free-breathing condition are almost identical to those obtained under expiratory condition. Nevertheless, care should be taken when analyzing PCASL data acquired under free-breathing. Especially in patients with shortness of breath, large changes in lung volume can lead to a reduction in the measured perfusion signal.Acknowledgements
No acknowledgement found.References
1. Ohno Y, et al. Dynamic perfusion MRI: Capability for evaluation of disease severity and progression of pulmonary arterial hypertension in patients with connective tissue disease. J Magn Reson Imaging 2008;28(4):887-899.
2. Tsuchiya N, et al. Decrease of pulmonary blood flow detected by phase contrast MRI is correlated with a decrease in lung volume and increase of lung fibrosis area determined by computed tomography in interstitial lung disease. Eur J Radiol 2016;85:1581-1585.
3. Sauter AW, et al. Multifunctional profiling of non-small cell lung cancer using 18F-FDG PET/CT and volume perfusion CT. J Nucl Med 2012;53:521-529.
4. Seith F, et al. Imaging pulmonary blood flow using pseudocontinuous arterial spin labeling (PCASL) with balanced steady-state free-precession (bSSFP) readout at 1.5T. J Magn Reson Imaging 2020;52:1767–1782.
5. Pengo MF, et al. Cardiorespiratory interaction with continuous positive airway pressure. J Thorac Dis 2018;10(Suppl 1):57-70.
6. Gilliam C, Küstner T, Blu T. 3D motion flow estimation using local all-pass filters. IEEE International Symposium on Biomedical Imaging (ISBI); 2016. p 282-285.
7. Küstner T, Schwartz M, Gatidis S, Schwenzer NF, Schmidt H, Yang B, Gilliam C, Blu T, Schick F. Local All-Pass Filter (LAP): Efficient Optical Flow-Based Image Registration. Proc ISMRM Workshop on Motion Correction in MRI and MRS; 2017.
Figures