2749
High spatio-temporal resolution 3D ASL renal perfusion with variable-density FSE and deep-learning reconstruction1Division of MRI research, Department of Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 2GE Research, Herzliya, Israel, 3Global MR Applications and Workflow, GE Healthcare, Boston, MA, United States, 4GE Research, Niskayuna, NY, United States
Synopsis
Arterial spin labeling (ASL) has proven to be a powerful research and clinical technique for functional imaging of tissues. The combination of undersampled acquisitions and compressed sensing reconstruction shows promise for increased speed, resolution, and robustness but conventional CS reconstructions are slow and may not be satisfactory, especially for low SNR data. This work explores the feasibility and performance of Deep-Learning based reconstruction of similarly sampled data to realize the full potential of these ASL acquisitions using DCI-net, an unenrolled iterative CS network. We show DCI-net performance at high acceleration rates and potential for fast volumetric ASL perfusion imaging.
Introduction
Arterial spin labeling (ASL) has proven to be a powerful research and clinical technique for functional imaging of tissues. The combination of undersampled acquisitions and compressed sensing (CS) reconstruction shows promise for increased speed, resolution, and robustness1–3, but conventional CS reconstructions are slow and may not be satisfactory, especially if regularization parameters are not tuned to the image SNR. This is a particular issue for ASL where signal-to-noise ratio (SNR) is modest and can be variable. This work explores the feasibility and performance of Deep-Learning (DL) based reconstruction of similarly sampled data to realize the full potential of these ASL acquisitions.Methods
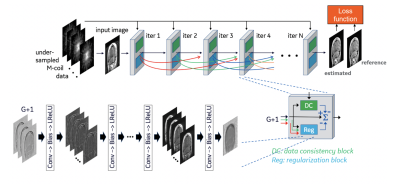
Data collection: Three healthy volunteers were scanned at 3T (GE Discovery MR750) using a 32-ch body array coil. We acquired paced-breathing volumetric perfusion volumes with a background-suppressed unbalanced PCASL preparation4 (1.5s/1.5s labeling/PLD, average B1=1.4μT, Gmax/Gav=3.5/0.5mT/m) and a variable-density FSE sequence (Poisson disk sampling, TR/TE=6000/10ms, ETL=120, k-space center oversampling followed by random distribution of phase/slice encodes in 20 excitations) collected in 4-5 min, with a net acceleration of R≈4.5. We also acquired a pre-saturated reference volume in 2 minutes without variable-density.Reconstruction: We use the DCI-net5,6, an unrolled iterative 2D image reconstruction network that consists of 28 iterative blocks and 20 dense skip-layer connections among iterations (Fig. 1). Each iteration includes a data-consistency and a convolutional unit. The former unit corrects for inconsistencies between the reconstructed and measured k-space data. The latter unit regularizes the reconstruction and is comprised of 9 2D convolutional layers with 96 kernels per layer.We trained three DCI-net models on retrospectively undersampled T1-weighted 3D brain scan data acquired using BRAVO (GE Healthcare). The undersampling was performed using variable-density Poisson-disc sampling patterns with different R values for each trained model. Specifically, we used R≈5, 8 and 14. As our loss function, we utilized a structural similarity index measure designed for complex-valued images between the reconstructed and ground truth data. The luminance, contrast and structure comparison functions had exponents 0.3, 1.0 and 0.3, respectively.5
We then reconstructed the acquired ASL volumes, but also 3 additional volumes formed by retrospectively undersampling with random excitation discarding with net accelerations of R≈8, 14 and extreme acceleration corresponding to a 2-shot FSE acquisition as this would fit in a single breath-hold scan (24s). External sensitivity maps calculated with ESPIRiT7 based on the reference volume were used in all reconstructions.
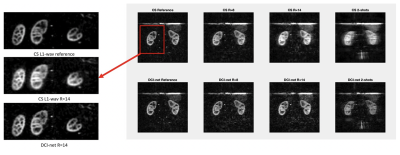
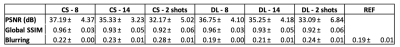
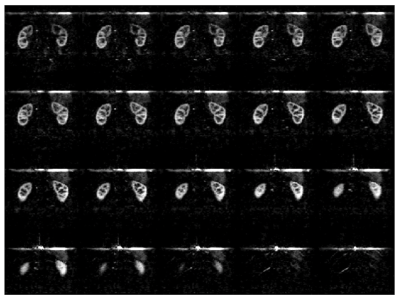
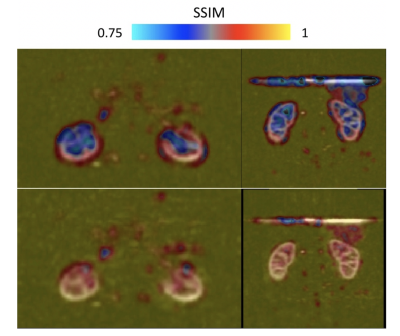
Image analysis: We chose the acquired dataset reconstructed with an L1-wavelet Compressed-Sensing (L1=0.001, 50 iterations) as implemented in the BART toolbox8 and previously reported as reference for further evaluation as this method has been previously validated1. We computed a structural-similarity-index (SSIM), as well as peak SNR (PSNR) between the different reconstructions and the reference. In addition, we calculated an SSIM map to provide visual quantitative insights into the differences between CS and DL reconstructions.
Results
Fast DL reconstructions using DCI-net were achieved on a standalone laptop (≈120 seconds per volume with a CPU-based inference on a MacBook Pro). Comparison between different reconstructions is shown in Fig. 2, highlighting the limits of L1-wavelet CS reconstructions, with image quality assessment metrics shown in Fig. 3. Compared to the reference, we can observe a degradation of image quality using CS that is not reflected in the DCI-net reconstructed volumes. As seen in Figs. 2 and 4, even at high R=14 acceleration corresponding to a minute-long scan, the DCI-net reconstructed volumes provide very good delineation of the corticomedullary renal structure albeit expected loss of SNR because of sampling density reduction. While this visual assessment isn’t particularly reflected in the SSIM metric, we observe a reduction of blurring when using DCI-net, as well as an increase in local SSIM in both kidneys as highlighted in the SSIM map shown in Fig. 5. It is worth mentioning that at extreme acceleration rates, both CS and DCI-net reconstructions provide significantly degraded volumes.Discussion/Conclusions
We have shown that DCI-net allows reconstructing highly accelerated renal perfusion volumes acquired with a variable-density FSE ASL sequence, enabling potential ≈1min whole kidney non-contrast perfusion scanning. Interestingly, this network has a great apparent robustness to image contrast and noise distribution by being trained on non-ASL data, aspects warranting further in-depth investigations. In contrast, the sampling density and pattern were found to be critical aspects of model training.Compared to iterative CS reconstruction, the DCI-net network has significant advantages including not requiring prior optimization of regularization parameters, faster reconstruction time implying ease of translation into imaging workflow and wider use of volumetric ASL, especially for renal applications which has seen increased traction9.
Acknowledgements
No acknowledgement found.References
1. Taso, M., Zhao, L., Guidon, A., Litwiller, D. V. & Alsop, D. C. Volumetric abdominal perfusion measurement using a pseudo-randomly sampled 3D fast-spin-echo (FSE) arterial spin labeling (ASL) sequence and compressed sensing reconstruction. Magnetic Resonance in Medicine 82, 680–692 (2019).
2. Munsch, F. et al. Rotated spiral RARE for high spatial and temporal resolution volumetric arterial spin labeling acquisition. NeuroImage 223, 117371 (2020).
3. Taso, M., Munsch, F., Zhao, L. & Alsop, D. C. Regional and depth-dependence of cortical blood-flow assessed with high-resolution Arterial Spin Labeling (ASL). Journal of Cerebral Blood Flow & Metabolism (2020).
4. Dai, W., Garcia, D., de Bazelaire, C. & Alsop, D. C. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 60, 1488–1497 (2008).
5. Ahn, S. et al. Contrast-weighted SSIM loss function for deep learning-based undersampled MRI reconstruction. in Proceedings of the 28th annual meeting of the ISMRM 1295 (2020).
6. Malkiel, I., Ahn, S., Slavens, Z., Taviani, V. & Hardy, C. J. Densely Connected Iterative Network for Sparse MRI Reconstruction. in Proceedings of the Joint annual meeting of the ISMRM-ESMRMB 3363 (2018).
7. Uecker, M. et al. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn. Reson. Med. 71, 990–1001 (2014).
8. Uecker, M. et al. Berkeley Advanced Reconstruction Toolbox. in Proc. Intl. Soc. Mag. Reson. Med 2486 (2015).
9. Nery, F. et al. Consensus-based technical recommendations for clinical translation of renal ASL MRI. Magn Reson Mater Phy 33, 141–161 (2020).
Figures