2743
Reduced Cerebral Blood Flow in Patients with Pulmonary Arterial Hypertension1Anesthesiology, University of California Los Angeles, Los Angeles, CA, United States, 2Medicine, University of California Los Angeles, Los Angeles, CA, United States, 3Bioengineering, University of California Los Angeles, Los Angeles, CA, United States, 4Radiological Sciences, University of California Los Angeles, Los Angeles, CA, United States, 5Brain Research Institute, University of California Los Angeles, Los Angeles, CA, United States
Synopsis
Pulmonary arterial hypertension (PAH) patients show cognitive and mood impairments, and brain tissue injury in those areas. However, the underlying cause of tissue damage in PAH patients remain unclear. Altered cerebral blood flow (CBF) may contribute to develop brain tissue injury and cognitive and mood deficits. We evaluated CBF in PAH patients over controls, and found changes in the prefrontal cortices, insula, cingulate, frontal cortex, corona radiate, temporal, occipital, and parietal gyrus. Significant correlations also emerged between CBF and functional deficits in PAH, including mood and cognition symptoms, implying altered hemodynamic contributing to brain changes and functional deficits.
INTRODUCTION
Patients with pulmonary arterial hypertension (PAH) frequently emerge with mood, autonomic, and cognitive aberrations,1-4 as well as significant brain changes appear in those regulatory areas, which may result from altered cerebral blood flow (CBF). However, regional CBF changes across the brain in PAH patients are unknown. Altered CBF may contribute to localized brain tissue injury reflected as cognitive and mood deficits, but the relationships between hemodynamic alterations and mood and cognitive symptoms are unknown in PAH patients. Regional brain CBF can be assessed by MRI based arterial spin labeling (ASL) imaging procedures, a non-invasive approach, without use of radiation or contrast agents for assessment of regional brain perfusion. ASL-based CBF values have been validated with positron emission tomography, and shown to be reproducible, making it favorable for regional CBF evaluation in PAH patients. Our aim was to examine regional brain CBF changes in PAH patients over age- and sex- matched control subjects using 3D pseudo-continuous ASL procedures, and evaluate the relationships between cognitive and mood scores and regional CBF values in PAH patients.METHODS
We examined 8 PAH [age, 47.0±12.0 years; body-mass-index (BMI), 29.4±10.0 kg/m2; 5 female] and 12 healthy control subjects (age, 56.0±8.0 years; BMI, 24.9±3.4 kg/m2; 7 female) using a 3.0-Tesla MRI (Siemens, Magnetom, Prisma Fit). Cognition, depressive, and anxiety symptoms were examined using the Montreal cognitive assessment (MoCA), Beck Depression Inventory (BDI-II), and Beck Anxiety Inventory (BAI), respectively in PAH and control subjects; scores were compared between groups (IBM SPSS; ANCOVA; covariates, age and sex). 3D pseudo-continuous ASL [pCASL] (TR = 4,000 ms, TE = 36.7 ms, FA = 120°, bandwidth = 2365 Hz/pixel, matrix size = 96×96, FOV = 240×240 mm, slice thickness = 2.5 mm) data were collected. Using the labeled and non-labeled ASL brain volumes, perfusion images were computed, and whole-brain CBF maps were generated. Whole-brain CBF maps were normalized to a common space, smoothed, and voxel-by-voxel CBF changes were assessed between groups (SPM12; ANCOVA; covariates, age and sex; p<0.05). Whole-brain CBF maps were correlated voxel-by-voxel with BAI, BDI-II, and MoCA scores in PAH patients using partial correlations (SPM12; covariates, age and sex, p<0.05). Brain clusters with significant differences between groups and correlation findings between CBF values and BAI, BDI-II, and MoCA scores were overlaid onto background images for structural identification.RESULTS
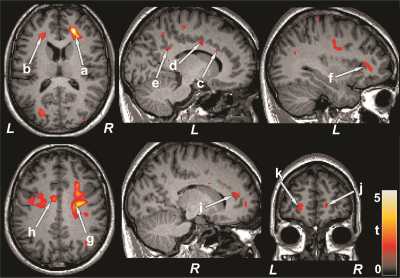
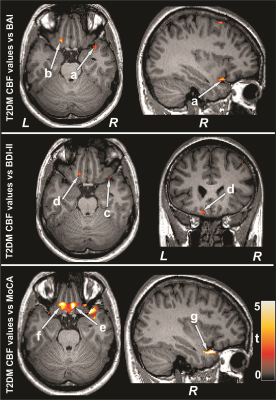
No significant differences in age, sex, or BMI appeared between groups (age, p=0.06; sex, p=0.85; BMI, p=0.26). PAH patients had significantly higher BAI (p=0.02) and lower global MoCA scores (p=0.008) compared to control subjects. However, BDI-II did not significantly differ between groups. Multiple brain areas showed reduced regional CBF values in PAH over control subjects (Fig. 1), including the prefrontal cortices, insula, cingulate, frontal cortex and white matter, corona radiate, temporal, occipital, and parietal gyrus and white matter. Anxiety scores showed negative associations with CBF values of the insula, basal forebrain, prefrontal and frontal cortices, and corona radiata in PAH subjects (Fig. 2). Negative relationships were observed in PAH subjects between depression scores and CBF values of the insula, basal forebrain, and frontal cortices (Fig. 2), and positive correlations emerged between MoCA scores and CBF values of the insula, basal-forebrain, and prefrontal and frontal cortices (Fig. 3).DISCUSSION
PAH patients showed significantly reduced CBF in multiple brain areas, including the prefrontal cortices, insula, cingulate, frontal cortex and white matter, corona radiate, temporal, occipital, and parietal gyrus and white matter over control subjects. Cognitive and mood scores were significantly correlated with region-specific CBF values in PAH patients. The findings suggest that brain injury in PAH patients may have stem from reduced CBF, and deficits in cognition and mood functions may result from altered hemodynamics in those regulatory sites in the condition. The pathophysiology underlying reduced CBF in PAH subjects may involve hyperventilation, cardiac right-to-left shunt, reduced cardiac output, and obstructed pulmonary artery flow.CONCLUSION
PAH patients exhibits reduced CBF that are associated with cognitive and mood dysfunctions in the condition. The findings indicate that the brain injury in PAH patient may have originated from altered hemodynamics and impacted cognitive and mood function in PAH patients.Acknowledgements
This work was supported by the UCLA Clinical and Translational Science Institute Research Scholar Award (UL1TR001881).References
1. White, J., Hopkins, R.O., Glissmeyer, E.W., Kitterman, N. & Elliott, C.G. Cognitive, emotional, and quality of life outcomes in patients with pulmonary arterial hypertension. Respiratory research 7: 55-, 2006.
2. Pagani-Estevez, G.L., Swetz, K.M., McGoon, M.D., Frantz, R.P., Tointon, S.K., Karnyski, A.M., Durst, L.A. & Watson, J.C. Characterization of Prostacyclin-associated Leg Pain in Patients with Pulmonary Arterial Hypertension. Ann Am Thorac Soc 14: 206-12, 2017.
3. Wensel, R., Jilek, C., Dorr, M., Francis, D.P., Stadler, H., Lange, T., Blumberg, F., Opitz, C., Pfeifer, M. & Ewert, R. Impaired cardiac autonomic control relates to disease severity in pulmonary hypertension. Eur Respir J 34: 895-901, 2009.
4. Bussotti, M. & Sommaruga, M. Anxiety and depression in patients with pulmonary hypertension: impact and management challenges. Vasc Health Risk Manag 14: 349-60, 2018.
Figures