2739
Brain perfusion in dementia with Parkinson's disease and Alzheimer’s disease: an arterial spin labeling MRI study1Dalian Medical University, Northern Jiangsu People’s Hospital, Yangzhou, China, Yangzhou, China, 2GE Healthcare, MR Research China, Beijing, P.R. China, Beijing, China
Synopsis
The present study was to explore regional perfusion changes in patients with Parkinson's disease dementia (PDD) and Alzheimer’s disease (AD) using 3D arterial spin labeling MRI and further assess the corresponding difference between PDD and AD patients. The results revealed that the perfusion pattern in PDD group was distinct from that in AD group despite of overlapped perfusion regions. The resultant normalized cerebral blood flow (CBF) can distinguish PDD from AD. Therefore, normalized CBF provided sensitive imaging-based markers that contribute to the diagnosis and differential diagnosis of the two dementia.
Introduction
Parkinson's disease (PD) is a progressive movement disorder characterized by loss of dopaminergic neurons in the substantia nigra. 10% of PD patients develop PD dementia (PDD)1 annually. In comparison, Alzheimer’s disease (AD) is characterized by the deposition of beta-amyloid (plaques) and tau protein (neurofibrillary tangles) as the central pathological features2. Cognitive impairment (CI) has been found in both AD and PDD patients. However, it remains difficult to differentiate both diseases based on clinical CI evaluation.3D arterial spin labeling (ASL) MRI, as a well-established method, is able to measure cerebral blood perfusion quantitatively. ASL derived absolute cerebral blood flow (CBF) has been widely applied to early diagnosis and evaluation of treatment effect for AD and PD patients. However, A recent study reported that a further normalized CBF is able to provide more sensitive perfusion pattern than absolute CBF, being comparable to PET-derived PD motor-related pattern (PDRP)3. Therefore, the main goal of this study was to investigate if normalized CBF was sufficiently sensitive to detect changes in perfusion between PDD and AD.
Materials and Methods
24 patients with AD (17 men and 7 women, mean age: 68.95±6.97 years), 26 patients with PDD (18 men and 8 women, mean age: 66.50±7.01) and 35 controls (18 men and 17 women, mean age: 62.75±3.50 years) underwent a routine brain MRI, 3D pseudo-continuous (pc)ASL and neuropsychological tests, including MMSE and MoCA for global cognitive functions. Moreover, memory, attention, executive function, visual space function, and language related tests in turn. The UPDRS-III and the H &Y stages were used to assess motor function of PDD patients.All MR imaging was acquired on a 3.0 Tesla (Discovery MR750, GE, USA) with 8 coil employed. 3D pcASL imaging was acquired with the following parameters: TR = 4844 ms, TE = 5.1 ms, post labeling delay = 2025 ms, FOV = 240 mm × 240 mm, matrix size = 64x64, 4 mm slice thickness and 0 mm gap slice space, NEX =3. The scan time was 4 minutes 41 seconds. For anatomic location and volume correction, a high-resolution 3D anatomical T1 weighted brain volume imaging (3D-BRAVO) were collected.
Data analysis 3D pcASL data were analyzed using a vendor-provided post-processing software in GE ADW4.6 workstation. The whole brain CBF parametric mapping was obtained accordingly for each subject. The CBF maps and T1 images were preprocessed using SPM 12 software. After registration, normalization and smoothing, absolute CBF maps are obtained. Normalized CBF maps were obtained by dividing the CBF maps by the subject-specific whole brain mean CBF value for each subject. ANCOVA analysis was conducted on the absolute and normalized CBF maps of AD, PDD, and HC with age, gender, education level, and gray matter volumes as covariates, followed by post hoc two sample t tests. Then, between group two sample t tests were performed within the mask showing conspicuous differences acquired from ANCOVA analysis (P < 0.001, FWE corrected for multiple comparisons).
We also correlated regional CBF values with cognitive and motor assessment scores, and all significant brain areas were among the changed results between AD group and PDD group. All statistical analyses were performed in SPSS 25.0 software, the significance threshold of correlation analysis was set at P <0.05. Receiver operating characteristic (ROC) analysis was constructed on MedCalc 15.6 software to ascertain the recognition capacity efficacy of normalized CBF to discriminate AD from PDD patients.
Results
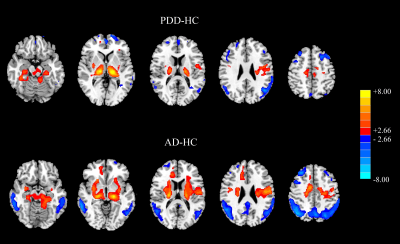
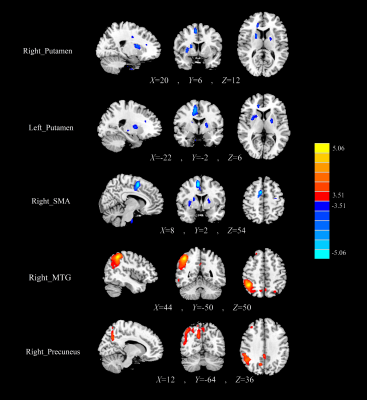
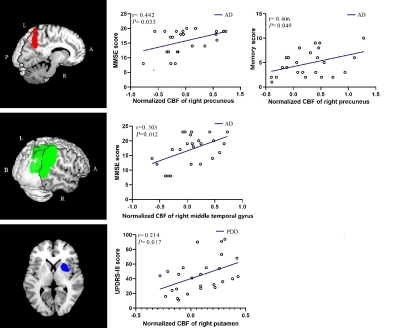
There was no evidence of absolute CBF difference among three groups. Normalized CBF reflected overlapped patterns of cortical hypoperfusion and subcortical hyperperfusion between AD and PDD group (Fig 1). AD patients had more extensive perfusion deficits in cognitive related areas (right middle temporal gyrus (MTG) and precuneus) than PDD patients. As compared with AD patients, the hypoperfusion of PDD patients mainly located in the subcortical and motor-related brain regions (right (supplementary motor area) SMA and bilateral putamen) (Fig 2).In AD group, the MMSE scores were positively correlated with the normalized CBF of the right precuneus and right middle temporal gyrus (P=0.033, r=0.442; P=0.012, r=0.503), and the memory scores were positively correlated with the normalized CBF of the right precuneus (P=0.049,r=0.406). The UPDRS-III scores showed positive relationship with normalized CBF of the right putamen (P=0.017, r=0.214) in PDD group (Fig 3). ROC analysis showed normalized CBF had good sensitivity and specificity to discriminate AD patients from PDD patients (Fig 4).
Discussion and conclusion
The results are consistent with previous studies4, no statistically significance of perfusion difference between AD and PDD was found by using absolute CBF. However, the regional perfusion patterns of AD and PDD using normalized CBF was in accordance with the identified patterns using spatial covariance analysis of 18F-fluorodeoxyglucose (FDG)-PET5,6.The significant difference of normalized CBF values in specific regions between the two groups, and significant correlations of normalized CBF values and characteristics scores in these regions were revealed in our study.Therefore, the normalized CBF might be considered to have clinical potential to explore functional changes between AD and PDD patients and reflect the perfusion differences of specific brain regions in corresponding to their own pathological changes between diseases.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant NO: 81471642). We greatly thank Dr. Songan Shang and Weiyin Vivian Liu for their comments on the manuscript writing, and Dr. Hongying Zhang for her methodological guidance.References
1. GOETZ CG, EMRE M, DUBOIS B. Parkinson's disease dementia: Definitions, guidelines, and research perspectives in diagnosis [J]. Annals of Neurology, 2010, 64.
2. LONG J, HOLTZMAN D. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies [J]. Cell, 2019, 179: 312-339.
3. S R, N K, J O, et al. Arterial spin labeling detects perfusion patterns related to motor symptoms in Parkinson's disease [J]. Parkinsonism & related disorders, 2020, 76: 21-28.
4. LE HERON CJ, WRIGHT SL, MELZER TR, et al. Comparing cerebral perfusion in Alzheimer's disease and Parkinson's disease dementia: an ASL-MRI study [J]. J Cereb Blood Flow Metab, 2014, 34: 964-970. 5. HABECK C, FOSTER N, PERNECZKY R, et al. Multivariate and univariate neuroimaging biomarkers of Alzheimer's disease [J]. NeuroImage, 2008, 40: 1503-1515.
6. MA Y, TANG C, SPETSIERIS P, et al. Abnormal metabolic network activity in Parkinson's disease: test-retest reproducibility [J]. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 2007, 27: 597-605.
Figures