2737
Robust Implementation of a 3D Pulsed ASL Sequence for Assessment of Liver Perfusion1Fraunhofer MEVIS, Bremen, Germany, 2University of Bremen, Bremen, Germany
Synopsis
Liver perfusion can give valuable functional information about diseases like carcinoma and cirrhosis. Arterial Spin Labeling allows non-invasive assessment of liver perfusion without exogenous contrast agents being especially helpful in patients with renal failure. ASL in abdominal organs like liver faces increased challenges due to low perfusion rates, breathing motion and strong off-resonance as well as B1-inhomogeneity. In this work, a robust implementation of a pulsed ASL (PASL) sequence with FAIR labeling and 3D GRASE readout is provided, addressing these difficulties.
Introduction
Liver perfusion can give valuable functional information about diseases like carcinoma and cirrhosis [1]. Arterial spin labeling (ASL) magnetic resonance imaging (MRI) allows non-invasive measurements of CBF by magnetically inverting inflowing blood, utilizing it as endogenous tracer. ASL is helpful in patients with renal failure since no contrast agent is needed. In addition, recent discussion about effects of deposition of gadolinium-based contrast agents in brain increases the potential of ASL as an alternative in clinical routine [2-4]. ASL in abdominal organs is challenging due to breathing motion and low perfusion rates requiring the acquisition of multiple averages. Good suppression of static tissue call for robust inversion pulses despite strong off-resonances and B1-inhomogeneity [5]. In this work, these difficulties are addressed by providing a robust implementation of a pulsed ASL (PASL) sequence with FAIR labeling and 3D GRASE readout for assessment of three-dimensional liver perfusion.Methods
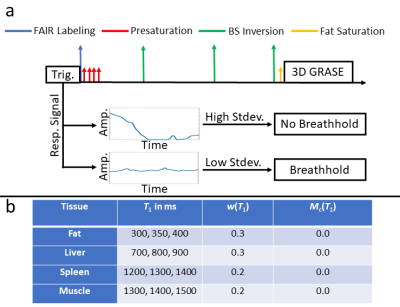
A FAIR PASL sequence is implemented as shown in Fig. 1a. A triggering mode is proposed where labeling and image acquisition is only played out if a breathhold is detected which will be referred to as multi-breathhold. This allows subjects to freely breath between subsequent acquisitions without following a specific breathing pattern as applied in previous studies [6].Background Suppression (BS)
A novel algorithm is proposed to find optimal timings of inversion pulses based on ranges of T1 values for specific tissue to increase the robustness against subject-specific variation. Therefore, the functional $$e(\vec{t})=\sum_{T_\text{1}}\left[\frac{M_\text{z}(T_\text{1},TI,\vec{t})}{M_\text{0}(T_\text{1})}-M_c(T_\text{1})\right]\cdot w(T_\text{1})(1)$$ is minimized. $$$\frac{M_\text{z}(T_\text{1},TI,\vec{t})}{M_\text{0}(T_\text{1})}$$$ is the longitudinal magnetization after inversion time $$$TI$$$ for tissue with relaxation time T1, $$$M_\text{c}(T_\text{1})$$$ is the desired magnetization at excitation, $$$w(T_\text{1})$$$ is a user defined weighting factor and $$$\vec{t}$$$ are the timings of the inversion pulses. Selected BS parameters are given in Fig. 1b.
Inversion Pulses
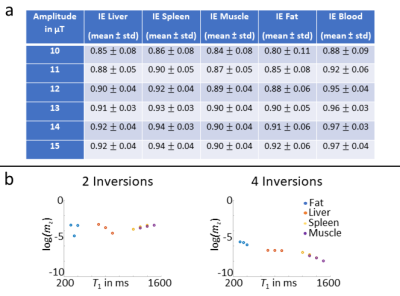
As described in [7], high efficiency of inversion pulses is important to prevent loss of perfusion signal. A c-shape FOCI inversion pulse with a modulation function of 20, β=790Hz and μ=6.8 is therefore used for FAIR labeling and BS [8]. Theoretical inversion efficiency for different tissue is calculated and incorporated in the BS algorithm (cf. Fig. 2a) for increased accuracy of timings. An amplitude of 13μT is selected as a compromise between SAR and inversion efficiency.
Experiments
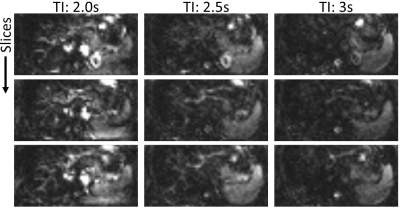
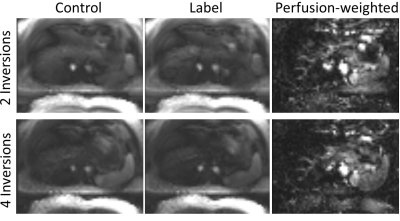
Perfusion-weighted images are obtained from a healthy female volunteer on a 3T Siemens Skyra Magnetom. Twenty label/control pairs are acquired with a bolus length of BL=2s and inflow times TI=[2s; 2.5s; 3s]. BS is applied with two inversions and multi-breathhold triggering is used. An additional experiment using four inversions and TI=2s with multi-breathhold is performed to investigate the effects of increased numbers of inversion pulses. Finally, data is acquired using TI=2s and two inversions under free-breathing conditions as performed in previous studies [9]. Imaging parameters of the 3D GRASE module were: Matrix 64x48x16, FoV: (300x225x80)mm3, Resolution: (4.7x4.7x5)mm3, Bandwidth: 3004Hz/Pixel, TR/TE: (6000ms/25.7ms).
Results
Perfusion-weighted images for different TIs are shown in Fig. 3. Fig. 4 shows the effect of the number of inversion pulses on reconstructed images. A comparison of perfusion-weighted images with multi-breathold/free-breathing acquisition is shown in Fig. 5.Discussion
Dynamics of liver perfusion are observed from different TIs (cf. Fig. 3). Early timepoints show macrovascular perfusion while microvascular components are visible for later inflows. Although global labeling is applied by the FAIR inversion pulse, perfusion signal appears quite low, indicating that arterial components are mainly observed. This is most likely related to the axial imaging plane used in this study. Hence, global perfusion could be observed by coronal or sagittal imaging planes. In addition, pseudo-continuous ASL (pCASL) allows more flexible labeling of arterial and portal-venous inflow and will be used in further studies. Typical strong perfusion signal from spleen and kidney is also observed, indicating effective labeling by the FOCI inversion pulse.Control and label images in Fig. 4 show good suppression of static tissue signal by using the proposed BS with two inversion pulses. Increased suppression is achieved with four pulses corresponding well with theoretical calculations (cf. Fig. 2b). Reduction of artifacts is also observed in perfusion-weighted images while maintaining perfusion signal, also indicating high inversion efficiency. Shifted fat signal is still visible using two and four inversions, being an indication for imperfect assumptions in the algorithm. The main cause is poor presaturation of fatty tissue due to off-resonance and non-adiabatic saturation pulses. The inclusion of imperfect presaturation in the BS scheme will further be investigated.
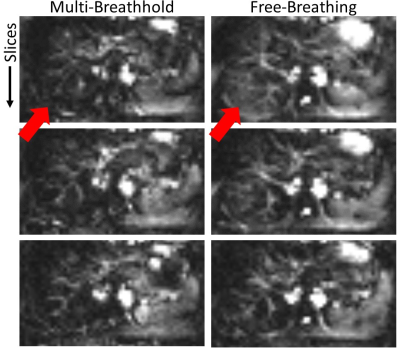
The comparison between multi-breathhold and free-breathing experiments reveal increased subtraction errors for the latter (cf. Fig. 5 arrows). These subtraction errors are of minor intensity and thus could be interpreted as perfusion, indicating the importance for the multi-breathhold strategy in liver ASL. As free-breathing acquisition greatly enhances the patients comfort during the examination however, future work will focus on the implementation of retrospective as well as prospective motion correction in PASL/pCASL liver experiments.
Conclusion
An implementation of a robust sequence for assessement of liver perfusion using PASL has been proposed and experimentally validated. Exchange of the labeling module from PASL to pCASL will allow flexible labeling of arterial and portal-venous blood supplies. In addition, dedicated retrospective and prospective motion correction will enable assessement of liver perfusion under free-breathing conditions.Acknowledgements
Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – 446287025.References
[1] Pan et al., "Quantification of Liver Perfusion Using Multidelay Pseudocontinuous Arterial Spin Labeling", Journal of Magnetic Resonance Imaging 43, 2016.
[2] Kanda et al., "Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy", Radiology 276, 2015.
[3] McDonald et al., "Intracranial Gadolinium Deposition after Contrastenhanced MR Imaging", Radiology 275, 2015.
[4] Radbruch et al., "Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent", Radiology 275, 2015.
[5] Garcia et al., "Efficiency of inversion pulses for background suppressed arterial spin labeling", Magnetic Resonance in Medicine 25, 2005.
[6] Martirosian et al., "Spatial-temporal perfusion patterns of the human liver assessed by pseudo-continuous arterial spin labeling MRI", Zeitschrift für medizinische Physik 29, 2019.
[7] Alsop et al., "Recommended Implementation of Arterial Spin Labeled Perfusion MRI for Clinical Applications: A consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia", Magnetic Resonance in Medicine 73, 2015.
[8] Ordidge et al., "Frequency offset corrected inversion (FOCI) pulses for use in localized spectroscopy", Magnetic Resonance in Medicine 36, 1996.
[9] Martirosian et al., "3D Arterial Spin Labeling Imaging of Arterial and Portal-VenousPerfusion in Human Liver at 3Tesla under Free Breathing:Preliminary Results", Proc. Intl. Soc. Mag. Reson. Med. 27, 2019.
[10] Stanisz et al., "T1, T2 relaxation and magnetization transfer in tissue at 3T", Magnetic Resonance in Medicine 54, 2005
[11] Bazelaire et al., "MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results", Radiology 230, 2004
[12] Gold et al., "Musculoskeletal MRI at 3.0 T: relaxation times and image contrast", American Journal of Roentgenology 183, 2004
Figures